Journal Description
Antibiotics
Antibiotics
is an international, peer-reviewed, open access journal on all aspects of antibiotics, published monthly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q1 (Pharmacology & Pharmacy) / CiteScore - Q1 (General Pharmacology, Toxicology and Pharmaceutics)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 13.7 days after submission; acceptance to publication is undertaken in 2.5 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
4.8 (2022);
5-Year Impact Factor:
4.9 (2022)
Latest Articles
Endotoxin-Induced Sepsis on Ceftriaxone-Treated Rats’ Ventilatory Mechanics and Pharmacokinetics
Antibiotics 2024, 13(1), 83; https://doi.org/10.3390/antibiotics13010083 - 15 Jan 2024
Abstract
Sepsis can trigger acute respiratory distress syndrome (ARDS), which can lead to a series of physiological changes, modifying the effectiveness of therapy and culminating in death. For all experiments, male Wistar rats (200–250 g) were split into the following groups: control and sepsis-induced
[...] Read more.
Sepsis can trigger acute respiratory distress syndrome (ARDS), which can lead to a series of physiological changes, modifying the effectiveness of therapy and culminating in death. For all experiments, male Wistar rats (200–250 g) were split into the following groups: control and sepsis-induced by endotoxin lipopolysaccharide (LPS); the control group received only intraperitoneal saline or saline + CEF while the treated groups received ceftriaxone (CEF) (100 mg/kg) IP; previously or not with sepsis induction by LPS (1 mg/kg) IP. We evaluated respiratory mechanics, and alveolar bronchial lavage was collected for nitrite and vascular endothelial growth factor (VEGF) quantification and cell evaluation. For pharmacokinetic evaluation, two groups received ceftriaxone, one already exposed to LPS. Respiratory mechanics shows a decrease in total airway resistance, dissipation of viscous energy, and elastance of lung tissues in all sepsis-induced groups compared to the control group. VEGF and NOx values were higher in sepsis animals compared to the control group, and ceftriaxone was able to reduce both parameters. The pharmacokinetic parameters for ceftriaxone, such as bioavailability, absorption, and terminal half-life, were smaller in the sepsis-induced group than in the control group since clearance was higher in septic animals. Despite the pharmacokinetic changes, ceftriaxone showed a reduction in resistance in the airways. In addition, CEF lowers nitrite levels in the lungs and acts on their adverse effects, reflecting pharmacological therapy of the disease.
Full article
(This article belongs to the Special Issue Pharmacokinetics and Pharmacodynamics Evaluation in the Appropriate Use of Antibacterial Drugs)
►
Show Figures
Open AccessArticle
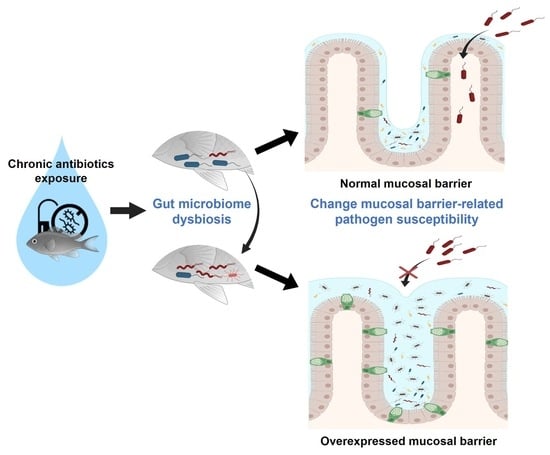
Effects of Antibiotic Residues on Fish Gut Microbiome Dysbiosis and Mucosal Barrier-Related Pathogen Susceptibility in Zebrafish Experimental Model
by
, , , , , , , and
Antibiotics 2024, 13(1), 82; https://doi.org/10.3390/antibiotics13010082 - 15 Jan 2024
Abstract
The symbiotic community of microorganisms in the gut plays an important role in the health of the host. While many previous studies have been performed on the interactions between the gut microbiome and the host in mammals, studies in fish are still lacking.
[...] Read more.
The symbiotic community of microorganisms in the gut plays an important role in the health of the host. While many previous studies have been performed on the interactions between the gut microbiome and the host in mammals, studies in fish are still lacking. In this study, we investigated changes in the intestinal microbiome and pathogen susceptibility of zebrafish (Danio rerio) following chronic antibiotics exposure. The chronic antibiotics exposure assay was performed on zebrafish for 30 days using oxytetracycline (Otc), sulfamethoxazole/trimethoprim (Smx/Tmp), or erythromycin (Ery), which are antibiotics widely used in the aquaculture industry. The microbiome analysis indicated that Fusobacteria, Proteobacteria, Firmicutes, and Bacteroidetes were the dominant phyla in the gut microbiome of the zebrafish used in this study. However, in Smx/Tmp-treated zebrafish, the compositions of Fusobacteria and Proteobacteria were changed significantly, and in Ery-treated zebrafish, the compositions of Proteobacteria and Firmicutes were altered significantly. Although alpha diversity analysis showed that there was no significant difference in the richness, beta diversity analysis revealed a community imbalance in the gut microbiome of all chronically antibiotics-exposed zebrafish. Intriguingly, in zebrafish with dysbiosis in the gut microbiome, the pathogen susceptibility to Edwardsiella piscicida, a representative Gram-negative fish pathogen, was reduced. Gut microbiome imbalance resulted in a higher count of goblet cells in intestinal tissue and an upregulation of genes related to the intestinal mucosal barrier. In addition, as innate immunity was enhanced by the increased mucosal barrier, immune and stress-related gene expression in the intestinal tissue was downregulated. In this study, we provide new insight into the effect of gut microbiome dysbiosis on pathogen susceptibility.
Full article
(This article belongs to the Special Issue Use of Antibiotics in Animals and Antimicrobial Resistance)
►▼
Show Figures
Graphical abstract
Open AccessArticle
In Vitro Antimicrobial Activity of Five Newly Approved Antibiotics against Carbapenemase-Producing Enterobacteria—A Pilot Study in Bulgaria
by
, , , , and
Antibiotics 2024, 13(1), 81; https://doi.org/10.3390/antibiotics13010081 - 15 Jan 2024
Abstract
To solve the problem with pan-drug resistant and extensively drug-resistant Gram-negative microbes, newly approved drugs such as ceftazidime/avibactam, cefiderocol, plazomicin, meropenem/vaborbactam, and eravacycline have been introduced in practice. The aim of the present study was to collect carbapenemase-producing clinical Enterobacterales isolates, to characterize
[...] Read more.
To solve the problem with pan-drug resistant and extensively drug-resistant Gram-negative microbes, newly approved drugs such as ceftazidime/avibactam, cefiderocol, plazomicin, meropenem/vaborbactam, and eravacycline have been introduced in practice. The aim of the present study was to collect carbapenemase-producing clinical Enterobacterales isolates, to characterize their carbapenemase genes and clonal relatedness, and to detect their susceptibility to commonly used antimicrobials and the above-mentioned newly approved antibiotics. Sixty-four carbapenemase producers were collected in a period of one year from four Bulgarian hospitals, mainly including Klebsiella pneumoniae (89% of the isolates) and also single Proteus mirabilis, Providencia stuartii and Citrobacter freundii isolates. The main genotype was blaNDM-1 (in 61%), followed by blaKPC-2 (23%), blaVIM-1 (7.8%) and blaOXA-48 (7.8%). Many isolates showed the presence of ESBL (blaCTX-M-15/-3 in 76.6%) and AmpC (blaCMY-4 in 37.5% or blaCMY-99 in 7.8% of isolates). The most common MLST type was K. pneumoniae ST11 (57.8%), followed by ST340 (12.5%), ST258 (6.3%) and ST101 (6.3%). The isolates were highly resistant to standard-group antibiotics, except they were susceptible to tigecycline (83.1%), colistin (79.7%), fosfomycin (32.8%), and aminoglycosides (20.3–35.9%). Among the newly approved compounds, plazomicin (90.6%) and eravacycline (76.3%) showed the best activity. Susceptibility to ceftazidime/avibactam and meropenem/vaborbactam was 34.4% and 27.6%, respectively. For cefiderocol, a large discrepancy was observed between the percentages of susceptible isolates according to EUCAST susceptibility breakpoints (37.5%) and those of CLSI (71.8%), detected by the disk diffusion method. This study is the first report to show patterns of susceptibility to five newly approved antibiotics among molecularly characterized isolates in Bulgaria. The data may contribute to both the improvement of treatment of individual patients and the choice of infection control strategy and antibiotic policy.
Full article
(This article belongs to the Topic Antimicrobial Agents and Nanomaterials)
Open AccessArticle
Extracellular Vesicles from a Biofilm of a Clinical Isolate of Candida albicans Negatively Impact on Klebsiella pneumoniae Adherence and Biofilm Formation
by
, , , , , , , , , and
Antibiotics 2024, 13(1), 80; https://doi.org/10.3390/antibiotics13010080 - 15 Jan 2024
Abstract
►▼
Show Figures
The opportunistic human fungal pathogen Candida albicans produces and releases into the surrounding medium extracellular vesicles (EVs), which are involved in some processes as communication between fungal cells and host–pathogen interactions during infection. Here, we have conducted the isolation of EVs produced by
[...] Read more.
The opportunistic human fungal pathogen Candida albicans produces and releases into the surrounding medium extracellular vesicles (EVs), which are involved in some processes as communication between fungal cells and host–pathogen interactions during infection. Here, we have conducted the isolation of EVs produced by a clinical isolate of C. albicans during biofilm formation and proved their effect towards the ability of the Gram-negative bacterial pathogen Klebsiella pneumoniae to adhere to HaCaT cells and form a biofilm in vitro. The results represent the first evidence of an antagonistic action of fungal EVs against bacteria.
Full article
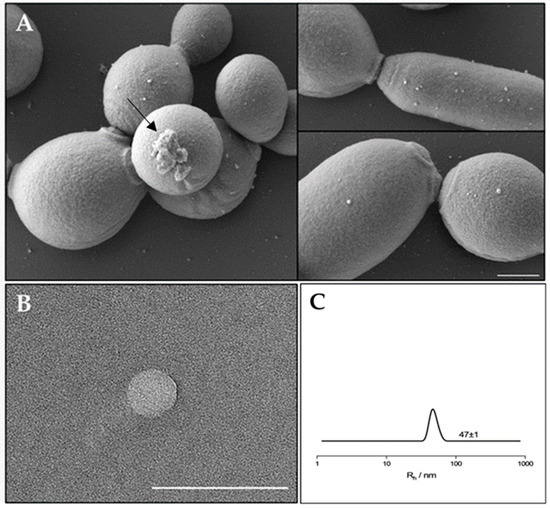
Figure 1
Open AccessArticle
Successful Clinical Avoidance of Colorectal Anastomotic Leakage through Local Decontamination
Antibiotics 2024, 13(1), 79; https://doi.org/10.3390/antibiotics13010079 - 15 Jan 2024
Abstract
Aim: An anastomotic leak is an unpredictable postoperative complication during recovery from colorectal surgery that may require a re-operation. Potentially pathogenic bacteria like Pseudomonas (and Enterococcus) contribute to the pathogenesis of an anastomotic leak through their capacity to degrade collagen and to activate
[...] Read more.
Aim: An anastomotic leak is an unpredictable postoperative complication during recovery from colorectal surgery that may require a re-operation. Potentially pathogenic bacteria like Pseudomonas (and Enterococcus) contribute to the pathogenesis of an anastomotic leak through their capacity to degrade collagen and to activate tissue matrix metalloprotease-9 in host intestinal tissues. The microbiome, therefore, is the key to preventing an anastomotic leak after colorectal surgery. The aim of this trial was to investigate whether perioperative selective decontamination with a new mixture of locally acting antibiotics specially designed against Pseudomonas aeruginosa and Enterococcus faecalis can reduce or even stop early symptomatic leakage. Method: All hospitalized patients in our University Clinic undergoing colorectal surgery with a left-sided anastomosis were included as two groups; patients in the intervention group received polymyxin B, gentamicin and vancomycin every six hours for five postoperative days and those in the control group did not receive such an intervention. An anastomotic leak was defined as a clinically obvious defect of the intestinal wall integrity at the colorectal anastomosis site (including suture) that leads to a communication between the intra- and extraluminal compartments, requiring a re-do surgery within seven postoperative days. Results: Between February 2017 and May 2023, a total of 301 patients (median age of 63 years) were analyzed. An anastomotic leak was observed in 11 patients in the control group (n = 152), but in no patients in the intervention group (n = 149); this difference was highly significant. Conclusion: The antibiotic mixture (with polymyxin B, gentamicin and vancomycin) used for local decontamination in our study stopped the occurrence of anastomotic leaks completely. According to the definition of anastomotic leak, no further surgery was required after local perioperative decontamination.
Full article
Open AccessArticle
Antibacterial and Antiviral Properties of Chenopodin-Derived Synthetic Peptides
by
, , , , , , , , , , , , , , , and
Antibiotics 2024, 13(1), 78; https://doi.org/10.3390/antibiotics13010078 - 14 Jan 2024
Abstract
Antimicrobial peptides have been developed based on plant-derived molecular scaffolds for the treatment of infectious diseases. Chenopodin is an abundant seed storage protein in quinoa, an Andean plant with high nutritional and therapeutic properties. Here, we used computer- and physicochemical-based strategies and designed
[...] Read more.
Antimicrobial peptides have been developed based on plant-derived molecular scaffolds for the treatment of infectious diseases. Chenopodin is an abundant seed storage protein in quinoa, an Andean plant with high nutritional and therapeutic properties. Here, we used computer- and physicochemical-based strategies and designed four peptides derived from the primary structure of Chenopodin. Two peptides reproduce natural fragments of 14 amino acids from Chenopodin, named Chen1 and Chen2, and two engineered peptides of the same length were designed based on the Chen1 sequence. The two amino acids of Chen1 containing amide side chains were replaced by arginine (ChenR) or tryptophan (ChenW) to generate engineered cationic and hydrophobic peptides. The evaluation of these 14-mer peptides on Staphylococcus aureus and Escherichia coli showed that Chen1 does not have antibacterial activity up to 512 µM against these strains, while other peptides exhibited antibacterial effects at lower concentrations. The chemical substitutions of glutamine and asparagine by amino acids with cationic or aromatic side chains significantly favoured their antibacterial effects. These peptides did not show significant hemolytic activity. The fluorescence microscopy analysis highlighted the membranolytic nature of Chenopodin-derived peptides. Using molecular dynamic simulations, we found that a pore is formed when multiple peptides are assembled in the membrane. Whereas, some of them form secondary structures when interacting with the membrane, allowing water translocations during the simulations. Finally, Chen2 and ChenR significantly reduced SARS-CoV-2 infection. These findings demonstrate that Chenopodin is a highly useful template for the design, engineering, and manufacturing of non-toxic, antibacterial, and antiviral peptides.
Full article
(This article belongs to the Special Issue The Century-Long Journey of Peptide-Based Drugs)
►▼
Show Figures
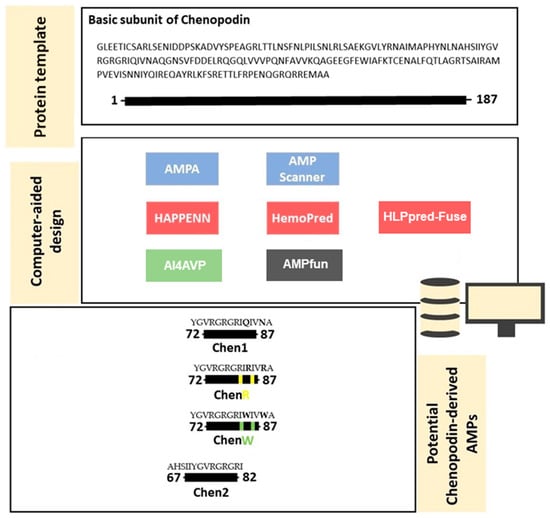
Figure 1
Open AccessReview
Innovative Techniques for Infection Control and Surveillance in Hospital Settings and Long-Term Care Facilities: A Scoping Review
by
, , , , , , , and
Antibiotics 2024, 13(1), 77; https://doi.org/10.3390/antibiotics13010077 - 13 Jan 2024
Abstract
Healthcare-associated infections (HAIs) pose significant challenges in healthcare systems, with preventable surveillance playing a crucial role. Traditional surveillance, although effective, is resource-intensive. The development of new technologies, such as artificial intelligence (AI), can support traditional surveillance in analysing an increasing amount of health
[...] Read more.
Healthcare-associated infections (HAIs) pose significant challenges in healthcare systems, with preventable surveillance playing a crucial role. Traditional surveillance, although effective, is resource-intensive. The development of new technologies, such as artificial intelligence (AI), can support traditional surveillance in analysing an increasing amount of health data or meeting patient needs. We conducted a scoping review, following the PRISMA-ScR guideline, searching for studies of new digital technologies applied to the surveillance, control, and prevention of HAIs in hospitals and LTCFs published from 2018 to 4 November 2023. The literature search yielded 1292 articles. After title/abstract screening and full-text screening, 43 articles were included. The mean study duration was 43.7 months. Surgical site infections (SSIs) were the most-investigated HAI and machine learning was the most-applied technology. Three main themes emerged from the thematic analysis: patient empowerment, workload reduction and cost reduction, and improved sensitivity and personalization. Comparative analysis between new technologies and traditional methods showed different population types, with machine learning methods examining larger populations for AI algorithm training. While digital tools show promise in HAI surveillance, especially for SSIs, challenges persist in resource distribution and interdisciplinary integration in healthcare settings, highlighting the need for ongoing development and implementation strategies.
Full article
(This article belongs to the Special Issue Antibacterial Resistance and Infection Control in ICU)
►▼
Show Figures
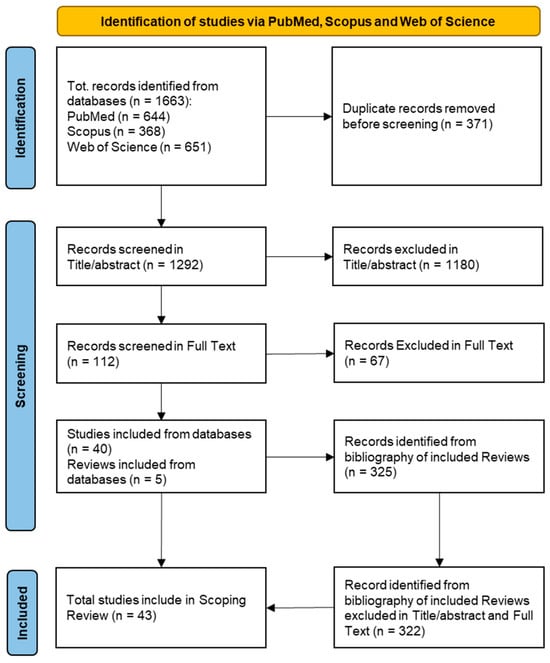
Figure 1
Open AccessReview
Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections
by
, , , , , , , , , , and
Antibiotics 2024, 13(1), 76; https://doi.org/10.3390/antibiotics13010076 - 13 Jan 2024
Abstract
Salmonella is a major foodborne pathogen and a leading cause of gastroenteritis in humans and animals. Salmonella is highly pathogenic and encompasses more than 2600 characterized serovars. The transmission of Salmonella to humans occurs through the farm-to-fork continuum and is commonly linked to
[...] Read more.
Salmonella is a major foodborne pathogen and a leading cause of gastroenteritis in humans and animals. Salmonella is highly pathogenic and encompasses more than 2600 characterized serovars. The transmission of Salmonella to humans occurs through the farm-to-fork continuum and is commonly linked to the consumption of animal-derived food products. Among these sources, poultry and poultry products are primary contributors, followed by beef, pork, fish, and non-animal-derived food such as fruits and vegetables. While antibiotics constitute the primary treatment for salmonellosis, the emergence of antibiotic resistance and the rise of multidrug-resistant (MDR) Salmonella strains have highlighted the urgency of developing antibiotic alternatives. Effective infection management necessitates a comprehensive understanding of the pathogen’s epidemiology and transmission dynamics. Therefore, this comprehensive review focuses on the epidemiology, sources of infection, risk factors, transmission dynamics, and the host range of Salmonella serotypes. This review also investigates the disease characteristics observed in both humans and animals, antibiotic resistance, pathogenesis, and potential strategies for treatment and control of salmonellosis, emphasizing the most recent antibiotic-alternative approaches for infection control.
Full article
(This article belongs to the Special Issue Novel Approaches for the Treatment of Antimicrobial-Resistant Pathogens)
►▼
Show Figures
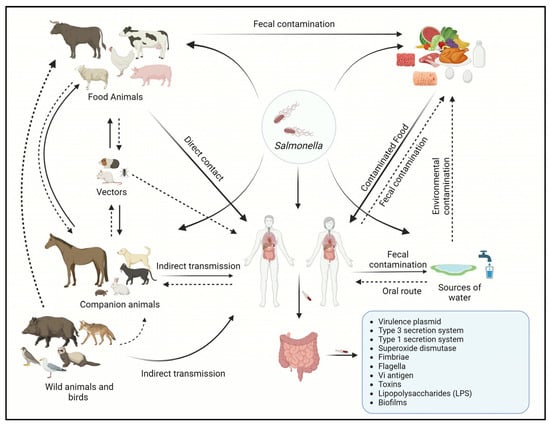
Figure 1
Open AccessReview
Small Schiff Base Molecules—A Possible Strategy to Combat Biofilm-Related Infections
Antibiotics 2024, 13(1), 75; https://doi.org/10.3390/antibiotics13010075 - 12 Jan 2024
Abstract
Microorganisms participating in the development of biofilms exhibit heightened resistance to antibiotic treatment, therefore infections involving biofilms have become a problem in recent years as they are more difficult to treat. Consequently, research efforts are directed towards identifying novel molecules that not only
[...] Read more.
Microorganisms participating in the development of biofilms exhibit heightened resistance to antibiotic treatment, therefore infections involving biofilms have become a problem in recent years as they are more difficult to treat. Consequently, research efforts are directed towards identifying novel molecules that not only possess antimicrobial properties but also demonstrate efficacy against biofilms. While numerous investigations have focused on antimicrobial capabilities of Schiff bases, their potential as antibiofilm agents remains largely unexplored. Thus, the objective of this article is to present a comprehensive overview of the existing scientific literature pertaining to small molecules categorized as Schiff bases with antibiofilm properties. The survey involved querying four databases (Web of Science, ScienceDirect, Scopus and Reaxys). Relevant articles published in the last 10 years were selected and categorized based on the molecular structure into two groups: classical Schiff bases and oximes and hydrazones. Despite the majority of studies indicating a moderate antibiofilm potential of Schiff bases, certain compounds exhibited a noteworthy effect, underscoring the significance of considering this type of molecular modeling when seeking to develop new molecules with antibiofilm effects.
Full article
(This article belongs to the Special Issue Organic Synthesis of Drug-Like Antimicrobial Compounds)
►▼
Show Figures
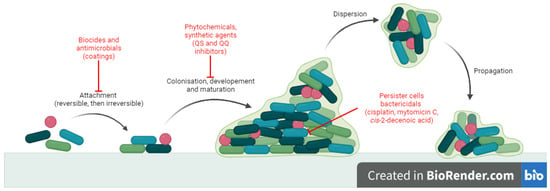
Figure 1
Open AccessArticle
Structures, Interactions and Activity of the N-Terminal Truncated Variants of Antimicrobial Peptide Thanatin
Antibiotics 2024, 13(1), 74; https://doi.org/10.3390/antibiotics13010074 - 12 Jan 2024
Abstract
Gram-negative bacteria are intrinsically more resistant to many frontline antibiotics, which is attributed to the permeability barrier of the outer membrane, drug efflux pumps and porins. Consequently, discovery of new small molecules antibiotics to kill drug-resistant Gram-negative bacteria presents a significant challenge. Thanatin,
[...] Read more.
Gram-negative bacteria are intrinsically more resistant to many frontline antibiotics, which is attributed to the permeability barrier of the outer membrane, drug efflux pumps and porins. Consequently, discovery of new small molecules antibiotics to kill drug-resistant Gram-negative bacteria presents a significant challenge. Thanatin, a 21-residue insect-derived antimicrobial peptide, is known for its potent activity against Enterobacter Gram-negative bacteria, including drug-resistant strains. Here, we investigated a 15-residue N-terminal truncated analog PM15 (P1IIYCNRRTGKCQRM15) of thanatin to determine modes of action and antibacterial activity. PM15 and the P1 to Y and A substituted variants PM15Y and PM15A delineated interactions and permeabilization of the LPS–outer membrane. In antibacterial assays, PM15 and the analogs showed growth inhibition of strains of Gram-negative bacteria that is largely dependent on the composition of the culture media. Atomic-resolution structures of PM15 and PM15Y in free solution and in complex with LPS micelle exhibited persistent β-hairpin structures similar to native thanatin. However, in complex with LPS, the structures of peptides are more compact, with extensive packing interactions among residues across the two anti-parallel strands of the β-hairpin. The docked complex of PM15/LPS revealed a parallel orientation of the peptide that may be sustained by potential ionic and van der Waals interactions with the lipid A moiety of LPS. Further, PM15 and PM15Y bind to LptAm, a monomeric functional variant of LptA, the periplasmic component of the seven-protein (A-G) complex involved in LPS transport. Taken together, the structures, target interactions and antibacterial effect of PM15 presented in the current study could be useful in designing thanatin-based peptide analogs.
Full article
(This article belongs to the Special Issue New Methodologies of Antibiotic Therapy: Drug Design and Diagnostic Tools)
►▼
Show Figures
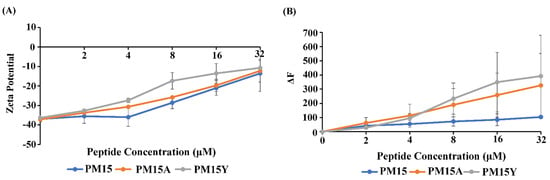
Figure 1
Open AccessArticle
Enhancing the Efficacy of Chloramphenicol Therapy for Escherichia coli by Targeting the Secondary Resistome
by
, , , , and
Antibiotics 2024, 13(1), 73; https://doi.org/10.3390/antibiotics13010073 - 12 Jan 2024
Abstract
►▼
Show Figures
The increasing prevalence of antimicrobial resistance and the limited availability of new antimicrobial agents have created an urgent need for new approaches to combat these issues. One such approach involves reevaluating the use of old antibiotics to ensure their appropriate usage and maximize
[...] Read more.
The increasing prevalence of antimicrobial resistance and the limited availability of new antimicrobial agents have created an urgent need for new approaches to combat these issues. One such approach involves reevaluating the use of old antibiotics to ensure their appropriate usage and maximize their effectiveness, as older antibiotics could help alleviate the burden on newer agents. An example of such an antibiotic is chloramphenicol (CHL), which is rarely used due to its hematological toxicity. In the current study, we employed a previously published transposon mutant library in MG1655/pTF2::blaCTX-M-1, containing over 315,000 unique transposon insertions, to identify the genetic factors that play an important role during growth in the presence of CHL. The list of conditionally essential genes, collectively referred to as the secondary resistome (SR), included 67 genes. To validate our findings, we conducted gene knockout experiments on six genes: arcA, hfq, acrZ, cls, mdfA, and nlpI. Deleting these genes resulted in increased susceptibility to CHL as demonstrated by MIC estimations and growth experiments, suggesting that targeting the products encoded from these genes may reduce the dose of CHL needed for treatment and hence reduce the toxicity associated with CHL treatment. Thus, the gene products are indicated as targets for antibiotic adjuvants to favor the use of CHL in modern medicine.
Full article
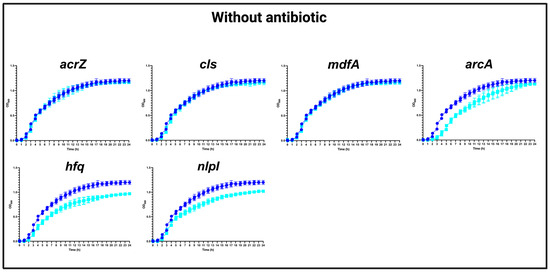
Figure 1
Open AccessReview
Translational PK/PD for the Development of Novel Antibiotics—A Drug Developer’s Perspective
by
, , , , , , and
Antibiotics 2024, 13(1), 72; https://doi.org/10.3390/antibiotics13010072 - 11 Jan 2024
Abstract
Antibiotic development traditionally involved large Phase 3 programs, preceded by Phase 2 studies. Recognizing the high unmet medical need for new antibiotics and, in some cases, challenges to conducting large clinical trials, regulators created a streamlined clinical development pathway in which a lean
[...] Read more.
Antibiotic development traditionally involved large Phase 3 programs, preceded by Phase 2 studies. Recognizing the high unmet medical need for new antibiotics and, in some cases, challenges to conducting large clinical trials, regulators created a streamlined clinical development pathway in which a lean clinical efficacy dataset is complemented by nonclinical data as supportive evidence of efficacy. In this context, translational Pharmacokinetic/Pharmacodynamic (PK/PD) plays a key role and is a major contributor to a “robust” nonclinical package. The classical PK/PD index approach, proven successful for established classes of antibiotics, is at the core of recent antibiotic approvals and the current antibacterial PK/PD guidelines by regulators. Nevertheless, in the case of novel antibiotics with a novel Mechanism of Action (MoA), there is no prior experience with the PK/PD index approach as the basis for translating nonclinical efficacy to clinical outcome, and additional nonclinical studies and PK/PD analyses might be considered to increase confidence. In this review, we discuss the value and limitations of the classical PK/PD approach and present potential risk mitigation activities, including the introduction of a semi-mechanism-based PK/PD modeling approach. We propose a general nonclinical PK/PD package from which drug developers might choose the studies most relevant for each individual candidate in order to build up a “robust” nonclinical PK/PD understanding.
Full article
(This article belongs to the Special Issue Featured Reviews in Pharmacokinetics and Pharmacodynamics of Antibiotics)
►▼
Show Figures
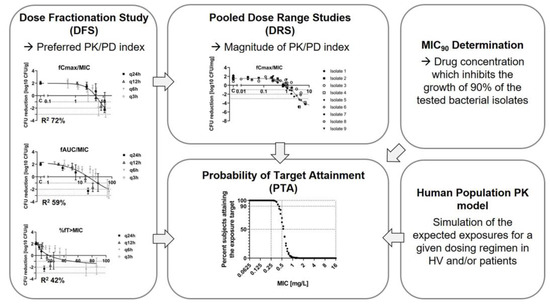
Figure 1
Open AccessReview
New Antimicrobial Strategies to Treat Multi-Drug Resistant Infections Caused by Gram-Negatives in Cystic Fibrosis
Antibiotics 2024, 13(1), 71; https://doi.org/10.3390/antibiotics13010071 - 11 Jan 2024
Abstract
People with cystic fibrosis (CF) suffer from recurrent bacterial infections which induce inflammation, lung tissue damage and failure of the respiratory system. Prolonged exposure to combinatorial antibiotic therapies triggers the appearance of multi-drug resistant (MDR) bacteria. The development of alternative antimicrobial strategies may
[...] Read more.
People with cystic fibrosis (CF) suffer from recurrent bacterial infections which induce inflammation, lung tissue damage and failure of the respiratory system. Prolonged exposure to combinatorial antibiotic therapies triggers the appearance of multi-drug resistant (MDR) bacteria. The development of alternative antimicrobial strategies may provide a way to mitigate antimicrobial resistance. Here we discuss different alternative approaches to the use of classic antibiotics: anti-virulence and anti-biofilm compounds which exert a low selective pressure; phage therapies that represent an alternative strategy with a high therapeutic potential; new methods helping antibiotics activity such as adjuvants; and antimicrobial peptides and nanoparticle formulations. Their mechanisms and in vitro and in vivo efficacy are described, in order to figure out a complete landscape of new alternative approaches to fight MDR Gram-negative CF pathogens.
Full article
(This article belongs to the Special Issue Novel Antimicrobial Strategies to Combat Multidrug-Resistant (MDR) Gram-Negative Bacteria)
►▼
Show Figures
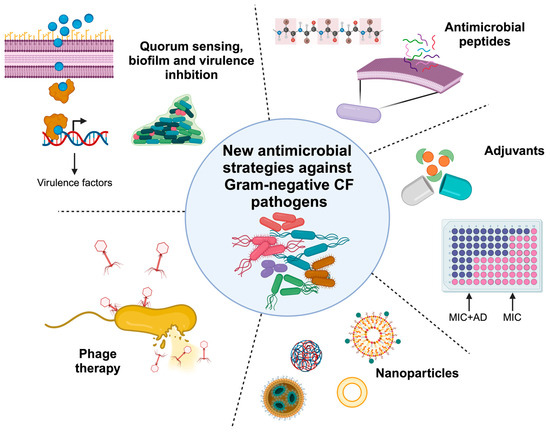
Figure 1
Open AccessArticle
Outpatient Antibiotic Prescribing Patterns in Children among Primary Healthcare Institutions in China: A Nationwide Retrospective Study, 2017–2019
by
, , , , , , , , and
Antibiotics 2024, 13(1), 70; https://doi.org/10.3390/antibiotics13010070 - 10 Jan 2024
Abstract
There is scarce evidence to demonstrate the pattern of antibiotic use in children in China. We aimed to describe antibiotic prescribing practices among children in primary healthcare institutions (PHIs) in China. We described outpatient antibiotic prescriptions for children in PHIs from January 2017
[...] Read more.
There is scarce evidence to demonstrate the pattern of antibiotic use in children in China. We aimed to describe antibiotic prescribing practices among children in primary healthcare institutions (PHIs) in China. We described outpatient antibiotic prescriptions for children in PHIs from January 2017 to December 2019 at both the national and diagnostic levels, utilizing the antibiotic prescribing rate (APR), multi-antibiotic prescribing rate (MAPR), and broad-spectrum prescribing rate (BAPR). Generalized estimating equations were adopted to analyze the factors associated with antibiotic use. Among the total 155,262.2 weighted prescriptions for children, the APR, MAPR, and BAPR were 43.5%, 9.9%, and 84.8%. At the national level, J01DC second-generation cephalosporins were the most prescribed antibiotic category (21.0%, N = 15,313.0), followed by J01DD third-generation cephalosporins (17.4%, N = 12,695.8). Watch group antibiotics accounted for 55.0% of the total antibiotic prescriptions (N = 52,056.3). At the diagnostic level, respiratory tract infections accounted for 67.4% of antibiotic prescriptions, among which prescriptions with diagnoses classified as potentially bacterial RTIs occupied the highest APR (55.0%). For each diagnostic category, the MAPR and BAPR varied. Age, region, and diagnostic categories were associated with antibiotic use. Concerns were raised regarding the appropriateness of antibiotic use, especially for broad-spectrum antibiotics.
Full article
(This article belongs to the Special Issue Antimicrobial Stewardship and Prescribing Practice)
►▼
Show Figures
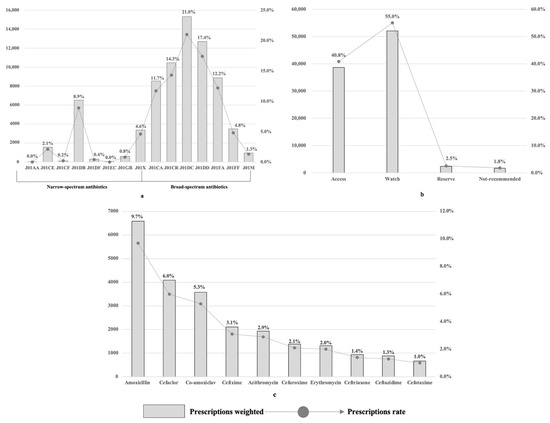
Figure 1
Open AccessSystematic Review
Antibiotic-Coated Intramedullary Nailing Managing Long Bone Infected Non-Unions: A Meta-Analysis of Comparative Studies
by
, , , , , , and
Antibiotics 2024, 13(1), 69; https://doi.org/10.3390/antibiotics13010069 - 10 Jan 2024
Abstract
Long bone infected non-unions are such an orthopedic challenge that antibiotic-coated intramedullary nailing (ACIN) has become a viable therapeutic option for their management. This study aims to provide a comprehensive assessment of the available data about the use of antibiotic-coated nailing in the
[...] Read more.
Long bone infected non-unions are such an orthopedic challenge that antibiotic-coated intramedullary nailing (ACIN) has become a viable therapeutic option for their management. This study aims to provide a comprehensive assessment of the available data about the use of antibiotic-coated nailing in the treatment of long bone infected non-unions. Following the PRISMA guideline in this meta-analysis, a systematic literature search was conducted across major databases for studies evaluating ACIN in long bone infected non-unions. The primary outcome measures included union rates, infection control, complications and functional status. Five eligible studies encompassing 183 patients in total met the inclusion criteria. The meta-analysis revealed no difference in the union rate in the antibiotic-coated intramedullary nailing group compared to that of the control group (OR = 1.73 [0.75–4.02]). Antibiotic-coated intramedullary nailing demonstrated no association with higher infection eradication (OR = 2.10 [0.97–4.54]). Also, functional outcome measure was mostly not significantly different between ACIN and control interventions. According to this meta-analysis, compared to the management of controls, ACIN is neither linked to increased union rates nor decreased infection rates. The paucity of research on this topic emphasizes the continuous need for additional well-designed randomized controlled trials for the application of antibiotics-coated intramedullary nailing in long bone non-unions.
Full article
(This article belongs to the Special Issue Advances in Orthopedic Infection Management and Antibiotic Treatment)
►▼
Show Figures
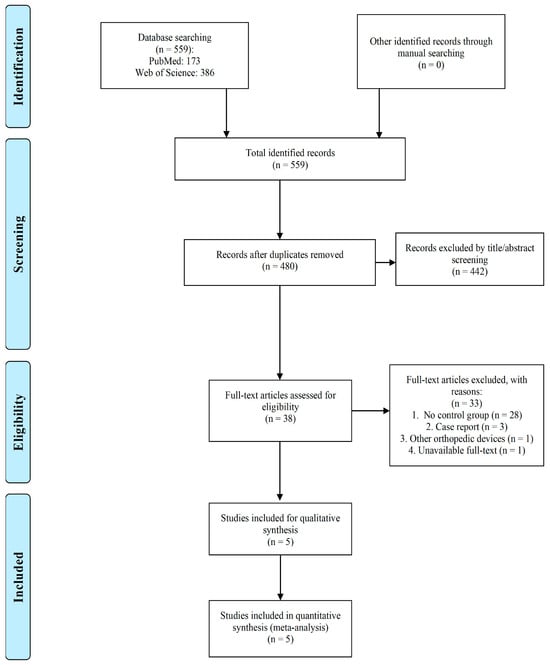
Figure 1
Open AccessArticle
Optimizing the Antimicrobial Activity of Sodium Hypochlorite (NaClO) over Exposure Time for the Control of Salmonella spp. In Vitro
by
, , , , and
Antibiotics 2024, 13(1), 68; https://doi.org/10.3390/antibiotics13010068 - 10 Jan 2024
Abstract
Fish is a nutritionally rich product; however, it is easily contaminated by pathogenic microorganisms, such as Salmonella spp. Therefore, this study aimed to identify the best concentration of sodium hypochlorite (NaClO), exposure time, and water temperature that allow the most effective antimicrobial effect
[...] Read more.
Fish is a nutritionally rich product; however, it is easily contaminated by pathogenic microorganisms, such as Salmonella spp. Therefore, this study aimed to identify the best concentration of sodium hypochlorite (NaClO), exposure time, and water temperature that allow the most effective antimicrobial effect on the viable population of Salmonella spp. Thus, Salmonella Enteritidis ATCC 13076 and Salmonella Schwarzengrund were exposed to different time frames, ranging from 5 min to 38.5 min, temperatures between 5 and 38.5 °C, and NaClO concentrations ranging from 0.36 to 6.36 ppm, through a central composite rotational design experiment (CCRD). The results demonstrated that the ATCC strain exhibited a quadratic response to sodium hypochlorite when combined with exposure time, indicating that initial contact would already be sufficient for the compound’s action to inhibit the growth of the mentioned bacteria. However, for S. Schwarzengrund (isolated directly from fish cultivated in aquaculture), both NaClO concentration and exposure time significantly influenced inactivation, following a linear pattern. This suggests that increasing the exposure time of NaClO could be an alternative to enhance Salmonella elimination rates in fish slaughterhouses. Thus, the analysis indicates that the Salmonella spp. strains used in in vitro experiments were sensitive to concentrations equal to or greater than the recommended ones, requiring a longer exposure time combined with the recommended NaClO concentration in the case of isolates from aquaculture.
Full article
(This article belongs to the Special Issue Characterization of Foodborne Pathogens: Antimicrobial Resistance, Genomic Aspects, Persistence and Virulence)
►▼
Show Figures
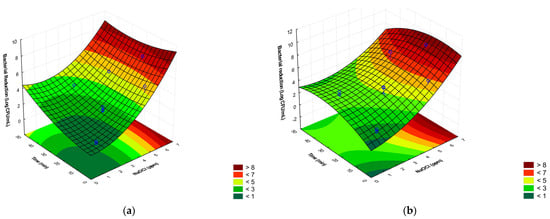
Figure 1
Open AccessBrief Report
Optimized Antibiotic Management of Critically Ill Patients with Severe Pneumonia Following Multiplex Polymerase Chain Reaction Testing: A Prospective Clinical Exploratory Trial
by
, , , , and
Antibiotics 2024, 13(1), 67; https://doi.org/10.3390/antibiotics13010067 - 10 Jan 2024
Abstract
Molecular diagnostic testing is assumed to enable fast respiratory pathogen identification and contribute to improved pneumonia management. We set up a prospective clinical trial at a tertiary hospital intensive care unit including adult patients suspected of severe pneumonia from whom a lower respiratory
[...] Read more.
Molecular diagnostic testing is assumed to enable fast respiratory pathogen identification and contribute to improved pneumonia management. We set up a prospective clinical trial at a tertiary hospital intensive care unit including adult patients suspected of severe pneumonia from whom a lower respiratory tract sample could be obtained. During control periods (CPs), routine testing was performed, and during intervention periods (IPs), this testing was completed with the FilmArray Pneumonia Panel plus test (FA-PNEU) executed 24/7. The main objective was to measure the impact of FA-PNEU results in terms of reduced time to targeted antimicrobial treatment administration. Over a 10-month period, analysis was performed on 35 CP and 50 IP patients. The median time to targeted antimicrobial treatment administration was reduced to 4.3 h in IPs compared to 26.4 h in CPs, with 54% of IP patients having FA-PNEU results that led to a treatment modification, of which all but one were targeted. Modifications included 10 (37%) de-escalations, 7 (25.9%) escalations, 3 (11.1%) regimen switches, and 7 (25.9%) complete antimicrobial discontinuations. FA-PNEU results were available with a 42.3 h gain compared to routine identification. This prospective study confirmed retrospective data demonstrating the benefit of FA-PNEU testing in severe pneumonia management of critically ill patients through improved antimicrobial use.
Full article
(This article belongs to the Special Issue Antimicrobial Stewardship in Critical Care)
►▼
Show Figures
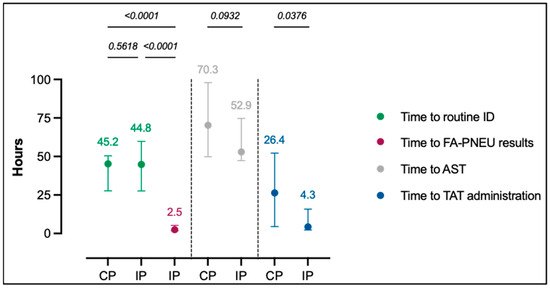
Figure 1
Open AccessReview
Antimicrobial Resistance in Streptococcus pneumoniae before and after the Introduction of Pneumococcal Conjugate Vaccines in Brazil: A Systematic Review
by
, , , , and
Antibiotics 2024, 13(1), 66; https://doi.org/10.3390/antibiotics13010066 - 09 Jan 2024
Abstract
Streptococcus pneumoniae causes serious illnesses, such as pneumonia, bacteremia, and meningitis, mainly in immunocompromised individuals and those of extreme ages. Currently, pneumococcal conjugate vaccines (PCVs) are the best allies against pneumococcal diseases. In Brazil, the 10-valent and 13-valent PCVs have been available since
[...] Read more.
Streptococcus pneumoniae causes serious illnesses, such as pneumonia, bacteremia, and meningitis, mainly in immunocompromised individuals and those of extreme ages. Currently, pneumococcal conjugate vaccines (PCVs) are the best allies against pneumococcal diseases. In Brazil, the 10-valent and 13-valent PCVs have been available since 2010, but the threat of antimicrobial resistance persists and has been changing over time. We conducted a systematic review of the literature with works published since 2000, generating a parallel between susceptibility data on isolates recovered from colonization and invasive diseases before and after the implementation of PCVs for routine childhood use in Brazil. This systematic review was based on the Cochrane Handbook for Systematic Reviews of Interventions and Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA) guidelines. Despite the inclusion of PCVs at a large scale in the national territory, high frequencies of non-susceptibility to important drugs used in pneumococcal diseases are still observed, especially penicillin, as well as increasing resistance to macrolides. However, there are still drugs for which pneumococci have a comprehensive sensitivity profile.
Full article
(This article belongs to the Section Mechanism and Evolution of Antibiotic Resistance)
►▼
Show Figures
Figure 1
Open AccessArticle
Unveiling the Relationship between Ceftobiprole and High-Molecular-Mass (HMM) Penicillin-Binding Proteins (PBPs) in Enterococcus faecalis
by
, , , , , , and
Antibiotics 2024, 13(1), 65; https://doi.org/10.3390/antibiotics13010065 - 09 Jan 2024
Abstract
Low-affinity PBP4, historically linked to penicillin resistance in Enterococcus faecalis, may still have affinity for novel cephalosporins. Ceftobiprole (BPR) is a common therapeutic choice, even with PBP4-related overexpression and amino acid substitution due to mutations. Our study aims to explore the interaction
[...] Read more.
Low-affinity PBP4, historically linked to penicillin resistance in Enterococcus faecalis, may still have affinity for novel cephalosporins. Ceftobiprole (BPR) is a common therapeutic choice, even with PBP4-related overexpression and amino acid substitution due to mutations. Our study aims to explore the interaction between BPR and High-Molecular-Mass (HMM) low-reactive PBPs in Penicillin-Resistant-Ampicillin-Susceptible/Ceftobiprole Non-Susceptible (PRAS/BPR-NS) E. faecalis clinical isolates. We conducted competition assays examining class A and B HMM PBPs from four PRAS/BPR-NS E. faecalis strains using purified membrane proteins and fluorescent penicillin (Bocillin FL), in treated and untreated conditions. Interaction strength was assessed calculating the 50% inhibitory concentration (IC50) values for ceftobiprole, by analyzing fluorescence intensity trends. Due to its low affinity, PBP4 did not display significant acylation among all strains. Moreover, both PBP1a and PBP1b showed a similar insensitivity trend. Conversely, other PBPs showed IC50 values ranging from 1/2-fold to 4-fold MICs. Upon higher BPR concentrations, increased percentages of PBP4 inhibition were observed in all strains. Our results support the hypothesis that PBP4 is necessary but not sufficient for BPR resistance, changing the paradigm for enterococcal cephalosporin resistance. We hypothesize that cooperation between class B PBP4 and at least one bifunctional class A PBP could be required to synthesize peptidoglycan and promote growth.
Full article
(This article belongs to the Section Mechanism and Evolution of Antibiotic Resistance)
►▼
Show Figures
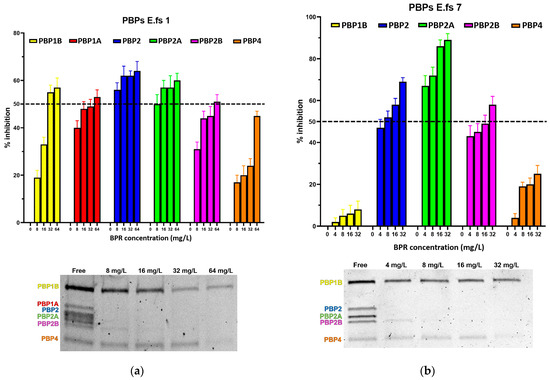
Figure 1
Open AccessFeature PaperArticle
Characterization of Antibiotic Treatment among Children Aged 0–59 Months Hospitalized for Acute Bacterial Gastroenteritis in Israel
Antibiotics 2024, 13(1), 64; https://doi.org/10.3390/antibiotics13010064 - 08 Jan 2024
Abstract
Background: We examined the extent and correlates of appropriate antibiotic use among children hospitalized with bacterial acute gastroenteritis (AGE) in Israel, a high-income country setting. Methods: Data were collected from children aged 0–59 months who participated in active hospital-based surveillance of AGE undertaken
[...] Read more.
Background: We examined the extent and correlates of appropriate antibiotic use among children hospitalized with bacterial acute gastroenteritis (AGE) in Israel, a high-income country setting. Methods: Data were collected from children aged 0–59 months who participated in active hospital-based surveillance of AGE undertaken during 2007–2015. Bacterial AGE was defined as having a positive stool culture for Salmonella, Shigella, Campylobacter, or dysentery. Appropriate antibiotic use was defined as the administration of ciprofloxacin, azithromycin, or third-generation cephalosporins during hospitalization or at discharge. Results: Overall, 550 children had bacterial AGE; of those, 369 (67.1% [95% CI 63.1–70.9]) received antibiotics, mostly azithromycin (61.8%) and third-generation cephalosporins (37.9%). Appropriate antibiotic treatment was given to 318/550 (57.8% [95% CI 53.7–61.9]). Children aged 0–11 months vs. 24–49 months were more likely to receive appropriate antibiotic treatment (OR = 1.90 [95% CI 1.09–3.33]). Having dysentery (OR = 5.30 [95% CI 3.35–8.39]), performing blood culture (OR = 1.59 [95% CI 1.02–2.48]), and C-reactive protein (CRP) levels (OR = 1.01 [95% CI 1.01–1.02]) were positively associated with receiving appropriate antibiotic treatment. Conclusions: Most children with bacterial AGE received appropriate antibiotic treatment, which correlated with young age, dysentery, CRP level, and performing blood culture, suggesting more severe illness, thus supporting the clinical decisions of physicians.
Full article
(This article belongs to the Special Issue Antibiotics Use and Therapy in Gram-Negative Bacterial Infection)
►▼
Show Figures
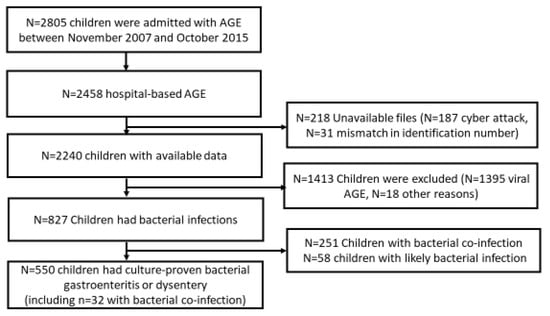
Figure 1

Journal Menu
► ▼ Journal Menu-
- Antibiotics Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Antibiotics, Antioxidants, JoF, Microbiology Research, Microorganisms
Redox in Microorganisms, 2nd Edition
Topic Editors: Michal Letek, Volker BehrendsDeadline: 31 July 2024
Topic in
Antibiotics, JPM, Pharmaceuticals, Pharmaceutics
Pharmacokinetic and Pharmacodynamic Modelling in Drug Discovery and Development
Topic Editors: Inaki F. Troconiz, Victor Mangas Sanjuán, Maria Garcia-Cremades MiraDeadline: 31 August 2024
Topic in
Molecules, Pharmaceutics, Antibiotics, Microorganisms, Biomolecules, Marine Drugs, Polymers, IJMS
Antimicrobial Agents and Nanomaterials
Topic Editors: Sandra Pinto, Vasco D. B. BonifácioDeadline: 30 September 2024
Topic in
Antibiotics, Biomedicines, JCM, Pharmaceuticals, Pharmaceutics
Challenges and Future Prospects of Antibacterial Therapy
Topic Editors: Kwang-sun Kim, Zehra EdisDeadline: 31 October 2024

Conferences
Special Issues
Special Issue in
Antibiotics
Antibiotic Therapy in Implant Related Orthopedic Infections
Guest Editor: Heinz WinklerDeadline: 20 January 2024
Special Issue in
Antibiotics
Staphylococcus aureus Skin and Soft Tissue Infections Pathogenesis and Therapeutic Approaches
Guest Editors: Marisa I. Gómez, Cintia González, Eliana CelaDeadline: 31 January 2024
Special Issue in
Antibiotics
Reviews on Antimicrobial Peptides
Guest Editor: Jean-Marc SabatierDeadline: 15 February 2024
Special Issue in
Antibiotics
Antimicrobial Resistance: What Can We Learn from Genomics?
Guest Editors: Ravi Kant, Tarja SironenDeadline: 29 February 2024
Topical Collections
Topical Collection in
Antibiotics
Antimicrobial Resistance and Anti-Biofilms
Collection Editors: Ding-Qiang Chen, Yulong Tan, Ren-You Gan, Guanggang Qu, Zhenbo Xu, Junyan Liu







.png)












