Journal Description
Pharmaceutics
Pharmaceutics
is a peer-reviewed, open access journal on the science and technology of pharmaceutics and biopharmaceutics, and is published monthly online by MDPI. The Spanish Society of Pharmaceutics and Pharmaceutical Technology (SEFIG), Pharmaceutical Solid State Research Cluster (PSSRC), Academy of Pharmaceutical Sciences (APS) and Korean Society of Pharmaceutical Sciences and Technology (KSPST) are affiliated with Pharmaceutics and their members receive a discount on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q1 (Pharmacology & Pharmacy) / CiteScore - Q1 (Pharmaceutical Science)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 14.2 days after submission; acceptance to publication is undertaken in 3.6 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
- Companion journal: Future Pharmacology
Impact Factor:
5.4 (2022);
5-Year Impact Factor:
6.0 (2022)
Latest Articles
Trans-Cinnamaldehyde—Fighting Streptococcus mutans Using Nature
Pharmaceutics 2024, 16(1), 113; https://doi.org/10.3390/pharmaceutics16010113 - 15 Jan 2024
Abstract
Streptococcus mutans (S. mutans) is the main cariogenic bacterium with acidophilic properties, in part due to its acid-producing and -resistant properties. As a result of this activity, hard tooth structures may demineralize and form caries. Trans-cinnamaldehyde (TC) is a phytochemical from
[...] Read more.
Streptococcus mutans (S. mutans) is the main cariogenic bacterium with acidophilic properties, in part due to its acid-producing and -resistant properties. As a result of this activity, hard tooth structures may demineralize and form caries. Trans-cinnamaldehyde (TC) is a phytochemical from the cinnamon plant that has established antibacterial properties for Gram-positive and -negative bacteria. This research sought to assess the antibacterial and antibiofilm effects of trans-cinnamaldehyde on S. mutans. TC was diluted to a concentration range of 156.25–5000 µg/mL in dimethyl sulfoxide (DMSO) 0.03–1%, an organic solvent. Antibacterial activity was monitored by testing the range of TC concentrations on 24 h planktonic growth compared with untreated S. mutans. The subminimal bactericidal concentrations (MBCs) were used to evaluate the bacterial distribution and morphology in the biofilms. Our in vitro data established a TC MBC of 2500 µg/mL against planktonic S. mutans using a microplate spectrophotometer. Furthermore, the DMSO-only controls showed no antibacterial effect against planktonic S. mutans. Next, the sub-MBC doses exhibited antibiofilm action at TC doses of ≥625 µg/mL on hydroxyapatite discs, as demonstrated through biofilm analysis using spinning-disk confocal microscopy (SDCM) and high-resolution scanning electron microscopy (HR-SEM). Our findings show that TC possesses potent antibacterial and antibiofilm properties against S. mutans. Our data insinuate that the most effective sub-MBC of TC to bestow these activities is 625 µg/mL.
Full article
(This article belongs to the Special Issue Biomaterials and Agents: Pharmaceutical and Biomedical Applications in Dental Research)
Open AccessArticle
Exploring the Ocular Absorption Pathway of Fasudil Hydrochloride towards Developing a Nanoparticulate Formulation with Improved Performance
Pharmaceutics 2024, 16(1), 112; https://doi.org/10.3390/pharmaceutics16010112 - 15 Jan 2024
Abstract
Rho-kinase (ROCK) inhibitors represent a new category of anti-glaucoma medications. Among them, Fasudil hydrochloride, a selective ROCK inhibitor, has demonstrated promising outcomes in glaucoma treatment. It works by inhibiting the ROCK pathway, which plays a crucial role in regulating the trabecular meshwork and
[...] Read more.
Rho-kinase (ROCK) inhibitors represent a new category of anti-glaucoma medications. Among them, Fasudil hydrochloride, a selective ROCK inhibitor, has demonstrated promising outcomes in glaucoma treatment. It works by inhibiting the ROCK pathway, which plays a crucial role in regulating the trabecular meshwork and canal of Schlemm’s aqueous humor outflow. This study aims to investigate the ocular absorption pathway of Fasudil hydrochloride and, subsequently, develop a nanoparticle-based delivery system for enhanced corneal absorption. Employing the ionic gelation method and statistical experimental design, the factors influencing chitosan nanoparticle (Cs NP) characteristics and performance were explored. Fasudil in vitro release and ex vivo permeation studies were performed, and Cs NP ocular tolerability and cytotoxicity on human lens epithelial cells were evaluated. Permeation studies on excised bovine eyes revealed significantly higher Fasudil permeation through the sclera compared to the cornea (370.0 μg/cm2 vs. 96.8 μg/cm2, respectively). The nanoparticle size (144.0 ± 15.6 nm to 835.9 ± 23.4 nm) and entrapment efficiency range achieved (17.2% to 41.4%) were predominantly influenced by chitosan quantity. Cs NPs showed a substantial improvement in the permeation of Fasudil via the cornea, along with slower release compared to the Fasudil aqueous solution. The results from the Hen’s Egg Test Chorioallantoic Membrane (HET-CAM) and Bovine Corneal Opacity and Permeability (BCOP) tests indicated good conjunctival and corneal biocompatibility of the formulated chitosan nanoparticles, respectively. Lens epithelial cells displayed excellent tolerance to low concentrations of these nanoparticles (>94% cell viability). To the best of our knowledge, this is the first report on the ocular absorption pathway of topically applied Fasudil hydrochloride where the cornea has been identified as a potential barrier that could be overcome using Cs NPs.
Full article
(This article belongs to the Special Issue Biofunctional Pharmaceutical Additives for Targeted, Improved Bioavailability and Safety of Medicine)
►▼
Show Figures
Figure 1
Open AccessReview
Emerging Oral Therapies for the Treatment of Psoriasis: A Review of Pipeline Agents
Pharmaceutics 2024, 16(1), 111; https://doi.org/10.3390/pharmaceutics16010111 - 15 Jan 2024
Abstract
The introduction of biologic agents for the treatment of psoriasis has revolutionized the current treatment landscape, targeting cytokines in the interleukin (IL)-23/IL-17 pathway and demonstrating strong efficacy and safety profiles in clinical trials. These agents however are costly, are associated with a risk
[...] Read more.
The introduction of biologic agents for the treatment of psoriasis has revolutionized the current treatment landscape, targeting cytokines in the interleukin (IL)-23/IL-17 pathway and demonstrating strong efficacy and safety profiles in clinical trials. These agents however are costly, are associated with a risk of immunogenicity, and require administration by intravenous or subcutaneous injection, limiting their use among patients. Oral therapies, specifically small molecule and microbiome therapeutics, have the potential to be more convenient and cost-effective agents for patients and have been a focus of development in recent years, with few targeted oral medications available for the disease. In this manuscript, we review pipeline oral therapies for psoriasis identified through a search of ClinicalTrials.gov (30 June 2022–1 October 2023). Available preclinical and clinical trial data on each therapeutic agent are discussed. Small molecules under development include tumor necrosis factor inhibitors, IL-23 inhibitors, IL-17 inhibitors, phosphodiesterase-4 inhibitors, Janus kinase inhibitors, A3 adenosine receptor agonists, and sphingosine-1-phosphate receptor 1 agonists, several of which are entering phase III trials. Oral microbials have also demonstrated success in early phase studies. As new oral therapies emerge for the treatment of psoriasis, real-world data and comparative trials are needed to better inform their use among patients.
Full article
(This article belongs to the Special Issue Current and Emerging Biological and Oral/Topical Small-Molecule Treatments for Psoriasis)
Open AccessArticle
Enhancing Liver Delivery of Gold Nanoclusters via Human Serum Albumin Encapsulation for Autoimmune Hepatitis Alleviation
by
, , , , , , , and
Pharmaceutics 2024, 16(1), 110; https://doi.org/10.3390/pharmaceutics16010110 - 14 Jan 2024
Abstract
Peptide-protected gold nanoclusters (AuNCs), possessing exceptional biocompatibility and remarkable physicochemical properties, have demonstrated intrinsic pharmaceutical activity in immunomodulation, making them a highly attractive frontier in the field of nanomedicine exploration. Autoimmune hepatitis (AIH) is a serious autoimmune liver disease caused by the disruption
[...] Read more.
Peptide-protected gold nanoclusters (AuNCs), possessing exceptional biocompatibility and remarkable physicochemical properties, have demonstrated intrinsic pharmaceutical activity in immunomodulation, making them a highly attractive frontier in the field of nanomedicine exploration. Autoimmune hepatitis (AIH) is a serious autoimmune liver disease caused by the disruption of immune balance, for which effective treatment options are still lacking. In this study, we initially identified glutathione (GSH)-protected AuNCs as a promising nanodrug candidate for AIH alleviating in a Concanavalin A (Con A)-induced mice model. However, to enhance treatment efficiency, liver-targeted delivery needs to be improved. Therefore, human serum albumin (HSA)-encapsulated AuNCs were constructed to achieve enhanced liver targeting and more potent mitigation of Con A-induced elevations in plasma aspartate transaminase (AST), alanine transaminase (ALT), and liver injury in mice. In vivo and in vitro mechanism studies indicated that AuNCs could suppress the secretion of IFN-γ by Con A-stimulated T cells and subsequently inhibit the activation of the JAK2/STAT1 pathway and eventual hepatocyte apoptosis induced by IFN-γ. These actions ultimately protect the liver from immune cell infiltration and damage caused by Con A. These findings suggest that bio-protected AuNCs hold promise as nanodrugs for AIH therapy, with their liver targeting capabilities and therapeutic efficiency being further improved via rational surface ligand engineering.
Full article
(This article belongs to the Special Issue Nanosystems for Drug Delivery)
►▼
Show Figures
Figure 1
Open AccessArticle
The Development of an Oral Solution Containing Nirmatrelvir and Ritonavir and Assessment of Its Pharmacokinetics and Stability
by
, , , , , , and
Pharmaceutics 2024, 16(1), 109; https://doi.org/10.3390/pharmaceutics16010109 - 14 Jan 2024
Abstract
Paxlovid®, a co-packaged medication comprised of separate tablets containing two active ingredients, nirmatrelvir (NRV) and ritonavir (RTV), exhibits good effectiveness against coronavirus disease 2019 (COVID-19). However, the size of the NRV/RTV tablets makes them difficult for some patients to swallow, especially
[...] Read more.
Paxlovid®, a co-packaged medication comprised of separate tablets containing two active ingredients, nirmatrelvir (NRV) and ritonavir (RTV), exhibits good effectiveness against coronavirus disease 2019 (COVID-19). However, the size of the NRV/RTV tablets makes them difficult for some patients to swallow, especially the elderly and those with dysphagia. Therefore, an oral liquid formulation that can overcome this shortcoming and improve patient compliance is required. In this study, we developed a liquid formulation containing NRV and RTV by adopting strategies that used co-solvents and surfactants to enhance the solubility and inhibit possible recrystallization. The in vitro release results showed that NRV and RTV could be maintained at high concentrations in solution for a certain period in the investigated media. In vivo studies in rats showed that the oral bioavailability of NRV/RTV solution was significantly enhanced. Compared to Paxlovid® tablets, the AUC(0–t) of NRV and RTV increased by 6.1 and 3.8 times, respectively, while the Cmax increased by 5.5 times for both. Furthermore, the promoting effect of the absorption of RTV on the bioavailability of NRV was confirmed. Experiments with a beagle showed a similar trend. Stability studies were also conducted at 4 °C, 25 °C, and 40 °C for 90 days, indicating that the oral liquid formulation was physically and chemically stable. This study can be used as a valuable resource for developing and applying oral liquid NRV/RTV formulations in a clinical context.
Full article
(This article belongs to the Special Issue Pharmacokinetics of Orally Administered Drugs, 2nd Edition)
►▼
Show Figures
Figure 1
Open AccessArticle
Long-Term Stability of Lavandula x intermedia Essential Oil Nanoemulsions: Can the Addition of the Ripening Inhibitor Impact the Biocidal Activity of the Nanoformulations?
by
, , , , , , , , and
Pharmaceutics 2024, 16(1), 108; https://doi.org/10.3390/pharmaceutics16010108 - 14 Jan 2024
Abstract
In this work, Lavandula x intermedia essential oil (LEO) was encapsulated in lipid-based nanoemulsions (NanoLEO) using the solvent-displacement technique. In order to preserve the colloidal stability of the formulation, LEO was appropriately doped with the incorporation of different levels of a water-insoluble oil
[...] Read more.
In this work, Lavandula x intermedia essential oil (LEO) was encapsulated in lipid-based nanoemulsions (NanoLEO) using the solvent-displacement technique. In order to preserve the colloidal stability of the formulation, LEO was appropriately doped with the incorporation of different levels of a water-insoluble oil used as a ripening inhibitor. All the nanoemulsion samples were evaluated in terms of the impact of the water-insoluble oil on the nanoemulsion formation, physical–chemical properties, and antibacterial effectiveness against E. coli (Gram-negative) and B. cereus (Gram-positive). The presence of the inert oil added benefits to the formulations in terms of appearance, colloidal stability, and loss of volatile components. However, the antimicrobial activity of the nanoemulsions dramatically decreased with the ripening inhibitor addition, probably because it hampered the internalization of the antimicrobial components of LEO within the bacterial cell membranes, thus nullifying the delivery ability of the nanoemulsion formulation. On the contrary, the undoped NanoLEO formulation showed unaltered antibacterial activity in both E. coli and B. cereus up to 40 weeks from the preparation.
Full article
(This article belongs to the Special Issue Essential Oils in Pharmaceutical Products (Volume II))
►▼
Show Figures
Figure 1
Open AccessArticle
Co-Delivery of a Novel Lipidated TLR7/8 Agonist and Hemagglutinin-Based Influenza Antigen Using Silica Nanoparticles Promotes Enhanced Immune Responses
by
, , , , , , , , and
Pharmaceutics 2024, 16(1), 107; https://doi.org/10.3390/pharmaceutics16010107 - 13 Jan 2024
Abstract
Co-delivery of antigens and adjuvants to the same antigen-presenting cells (APCs) can significantly improve the efficacy and safety profiles of vaccines. Here, we report amine-grafted silica nanoparticles (A-SNP) as a tunable vaccine co-delivery platform for TLR7/8 agonists along with the recombinant influenza antigen
[...] Read more.
Co-delivery of antigens and adjuvants to the same antigen-presenting cells (APCs) can significantly improve the efficacy and safety profiles of vaccines. Here, we report amine-grafted silica nanoparticles (A-SNP) as a tunable vaccine co-delivery platform for TLR7/8 agonists along with the recombinant influenza antigen hemagglutinin H7 (H7) to APCs. A-SNP of two different sizes (50 and 200 nm) were prepared and coated with INI-4001 at different coating densities, followed by co-adsorption of H7. Both INI-4001 and H7 showed >90% adsorption to the tested A-SNP formulations. TNF-α and IFN-α cytokine release by human peripheral blood mononuclear cells as well as TNF-α, IL-6, and IL-12 release by mouse bone marrow-derived dendritic cells revealed that the potency of the INI-4001-adsorbed A-SNP (INI-4001/A-SNP) formulations was improved relative to aqueous formulation control. This improved potency was dependent on particle size and ligand coating density. In addition, slow-release profiles of INI-4001 were measured from INI-4001/A-SNP formulations in plasma with 30–50% INI-4001 released after 7 days. In vivo murine immunization studies demonstrated significantly improved H7-specific humoral and Th1/Th17-polarized T cell immune responses with no observed adverse reactions. Low-density 50 nm INI-4001/A-SNP elicited significantly higher IFN-γ and IL-17 induction over that of the H7 antigen-only group and INI-4001 aqueous formulation controls. In summary, this work introduces an effective and biocompatible SNP-based co-delivery platform that enhances the immunogenicity of TLR7/8 agonist-adjuvanted subunit influenza vaccines.
Full article
(This article belongs to the Special Issue Innovative Drug Release and Vaccine Delivery Systems)
►▼
Show Figures
Graphical abstract
Open AccessArticle
Evaluation of the Drug-Induced Liver Injury Potential of Saxagliptin through Reactive Metabolite Identification in Rats
by
, , , , , and
Pharmaceutics 2024, 16(1), 106; https://doi.org/10.3390/pharmaceutics16010106 - 13 Jan 2024
Abstract
A liver injury was recently reported for saxagliptin, which is a dipeptidyl peptidase-4 (DPP-4) inhibitor. However, the underlying mechanisms of saxagliptin-induced liver injury remain unknown. This study aimed to evaluate whether saxagliptin, a potent and selective DPP-4 inhibitor that is globally used for
[...] Read more.
A liver injury was recently reported for saxagliptin, which is a dipeptidyl peptidase-4 (DPP-4) inhibitor. However, the underlying mechanisms of saxagliptin-induced liver injury remain unknown. This study aimed to evaluate whether saxagliptin, a potent and selective DPP-4 inhibitor that is globally used for treating type 2 diabetes mellitus, binds to the nucleophiles in vitro. Four DPP-4 inhibitors, including vildagliptin, were evaluated for comparison. Only saxagliptin and vildagliptin, which both contain a cyanopyrrolidine group, quickly reacted with L-cysteine to enzyme-independently produce thiazolinic acid metabolites. This saxagliptin–cysteine adduct was also found in saxagliptin-administered male Sprague–Dawley rats. In addition, this study newly identified cysteinyl glycine conjugates of saxagliptin and 5-hydroxysaxagliptin. The observed metabolic pathways were hydroxylation and conjugation with cysteine, glutathione, sulfate, and glucuronide. In summary, we determined four new thiazoline-containing thiol metabolites (cysteine and cysteinylglycine conjugates of saxagliptin and 5-hydroxysaxagliptin) in saxagliptin-administered male rats. Our results reveal that saxagliptin can covalently bind to the thiol groups of cysteine residues of endogenous proteins in vivo, indicating the potential for saxagliptin to cause drug-induced liver injury.
Full article
(This article belongs to the Special Issue Bioanalysis and Metabolomics, 2nd Edition)
►▼
Show Figures
Figure 1
Open AccessArticle
The Effect of Systemic Parameters and Baseline Characteristics in Short-Term Response Analysis with Intravitreal Ranibizumab in Treatment-Naive Patients with Neovascular Age-Related Macular Degeneration
by
, , , , , , , , , , and
Pharmaceutics 2024, 16(1), 105; https://doi.org/10.3390/pharmaceutics16010105 - 13 Jan 2024
Abstract
Anti-vascular endothelial growth factor drugs keep being the main therapy for neovascular age-related macular degeneration (AMD). Possible predictive parameters (demographic, biochemical and/or inflammatory) could anticipate short-term treatment response with ranibizumab. 46 treatment-naive patients were included in a prospective observational study. They underwent three
[...] Read more.
Anti-vascular endothelial growth factor drugs keep being the main therapy for neovascular age-related macular degeneration (AMD). Possible predictive parameters (demographic, biochemical and/or inflammatory) could anticipate short-term treatment response with ranibizumab. 46 treatment-naive patients were included in a prospective observational study. They underwent three monthly injections of intravitreal ranibizumab for neovascular AMD and the clinical examination was made at baseline and one month after the third injection. Demographic characteristics, co-morbidities and concomitant treatments were recorded at the baseline visit. Biochemical parameters, complete blood count and inflammation biomarkers were also measured at these times. Uric Acid was found to be statistically significant with a one-point difference between good and poor responders in both basal and treated patients, but only in basal parameters was statistical significance reached (p = 0.007 vs. p = 0.071 in treated patients). Cholesterol and inflammatory parameters such as white blood cell count and neutrophils were significantly reduced over time when treated with intravitreal ranibizumab. On the other hand, women seemed to have a worse prognosis for short-term response to intravitreal ranibizumab treatment. Uric acid may help identify possible non-responders before initial treatment with ranibizumab, and cholesterol and white blood cells could be good candidates to monitor short-term response to ranibizumab treatment.
Full article
(This article belongs to the Special Issue Unveiling New Insights and Treatment Options for Ocular Surface Diseases)
►▼
Show Figures
Figure 1
Open AccessArticle
Towards Precision Medicine in Clinical Practice: Alinity C vs. UHPLC-MS/MS in Plasma Aripiprazole Determination
by
, , , , , , , , and
Enrique Bandín-Vilar
Pharmaceutics 2024, 16(1), 104; https://doi.org/10.3390/pharmaceutics16010104 - 12 Jan 2024
Abstract
Therapeutic drug monitoring improves the benefit–risk balance of antipsychotic therapy. Ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) is considered the gold-standard method for measuring plasma drug concentrations; however, the Alinity C system has emerged as a promising alternative. This is the first study aimed
[...] Read more.
Therapeutic drug monitoring improves the benefit–risk balance of antipsychotic therapy. Ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) is considered the gold-standard method for measuring plasma drug concentrations; however, the Alinity C system has emerged as a promising alternative. This is the first study aimed at comparing UHPLC-MS/MS versus Alinity C in measuring plasma concentrations of aripiprazole and dehydroaripiprazole. A total of 86 plasma samples were analyzed. The active moiety of aripiprazole was measured in 60 samples using both systems and 26 samples were analyzed twice using Alinity C with an intermediate period of 6 months to assess its reproducibility. Spearman’s correlation revealed a good association between the two assays (rs = 0.96) and no significance differences were found by McNemar’s test when classifying samples between infra-, supra- and therapeutic ranges. Passing–Bablock regression showed a good correlation among methods (rs = 0.93) and a slope of 1.12 indicating a slight tendency of Alinity C to measure higher values than UHPLC-MS/MS. In addition, a good intra-method correlation across the two sequential analyses with Alinity C was obtained (rs = 0.99). Nonetheless, clinical decisions could be different in 15% of the cases depending on the chosen method. No differences were found in active moiety determination by Alinity C depending on the concentration of aripiprazole and dehydroaripiprazole of the samples.
Full article
(This article belongs to the Special Issue Therapeutic Drug Monitoring as a Useful Tool in Therapy Improvement, 2nd Edition)
►▼
Show Figures
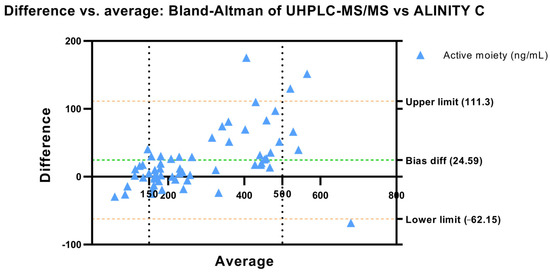
Figure 1
Open AccessReview
Assessment of In Vitro Release Testing Methods for Colloidal Drug Carriers: The Lack of Standardized Protocols
Pharmaceutics 2024, 16(1), 103; https://doi.org/10.3390/pharmaceutics16010103 - 12 Jan 2024
Abstract
Although colloidal carriers have been in the pipeline for nearly four decades, standardized methods for testing their drug-release properties remain to be established in pharmacopeias. The in vitro assessment of drug release from these colloidal carriers is one of the most important parameters
[...] Read more.
Although colloidal carriers have been in the pipeline for nearly four decades, standardized methods for testing their drug-release properties remain to be established in pharmacopeias. The in vitro assessment of drug release from these colloidal carriers is one of the most important parameters in the development and quality control of drug-loaded nano- and microcarriers. This lack of standardized protocols occurs due to the difficulties encountered in separating the released drug from the encapsulated one. This review aims to compare the most frequent types of release testing methods (i.e., membrane diffusion techniques, sample and separate methods and in situ detection techniques) in terms of the advantages and disadvantages of each one and of the key parameters that influence drug release in each case.
Full article
(This article belongs to the Special Issue Advances of Colloidal Systems in Drug Delivery and Therapeutic Application, 2nd Edition)
►▼
Show Figures
Graphical abstract
Open AccessArticle
Engineering Neurotoxin-Functionalized Exosomes for Targeted Delivery to the Peripheral Nervous System
by
, , , , , , and
Pharmaceutics 2024, 16(1), 102; https://doi.org/10.3390/pharmaceutics16010102 - 12 Jan 2024
Abstract
The administration of therapeutics to peripheral nerve tissue is challenging due to the complexities of peripheral neuroanatomy and the limitations imposed by the blood–nerve barrier (BNB). Therefore, there is a pressing need to enhance delivery effectiveness and implement targeted delivery methods. Recently, erythrocyte-derived
[...] Read more.
The administration of therapeutics to peripheral nerve tissue is challenging due to the complexities of peripheral neuroanatomy and the limitations imposed by the blood–nerve barrier (BNB). Therefore, there is a pressing need to enhance delivery effectiveness and implement targeted delivery methods. Recently, erythrocyte-derived exosomes (Exos) have gained widespread attention as biocompatible vehicles for therapeutics in clinical applications. However, engineering targeted Exos for the peripheral nervous system (PNS) is still challenging. This study aims to develop a targeted Exo delivery system specifically designed for presynaptic terminals of peripheral nerve tissue. The clostridium neurotoxin, tetanus toxin-C fragment (TTC), was tethered to the surface of red blood cell (RBC)-derived Exos via a facile and efficient bio-orthogonal click chemistry method without a catalyst. Additionally, Cyanine5 (Cy5), a reactive fluorescent tag, was also conjugated to track Exo movement in both in vitro and in vivo models. Subsequently, Neuro-2a, a mouse neuronal cell line, was treated with dye-labeled Exos with/without TTC in vitro, and the results indicated that TTC-Exos exhibited more efficient accumulation along the soma and axonal circumference, compared to their unmodified counterparts. Further investigation, using a mouse model, revealed that within 72 h of intramuscular administration, engineered TTC-Exos were successfully transported into the neuromuscular junction and sciatic nerve tissues. These results indicated that TTC played a crucial role in the Exo delivery system, improving the affinity to peripheral nerves. These promising results underscore the potential of using targeted Exo carriers to deliver therapeutics for treating peripheral neuropathies.
Full article
(This article belongs to the Special Issue Advances of Membrane Vesicles in Drug Delivery Systems, 2nd Edition)
►▼
Show Figures
Figure 1
Open AccessArticle
Preclinical Application of CEST MRI to Detect Early and Regional Tumor Response to Local Brain Tumor Treatment
by
, , , , , and
Pharmaceutics 2024, 16(1), 101; https://doi.org/10.3390/pharmaceutics16010101 - 12 Jan 2024
Abstract
►▼
Show Figures
Treating glioblastoma and monitoring treatment response non-invasively remain challenging. Here, we developed a robust approach using a drug-loaded liposomal hydrogel that is mechanically compatible with the brain, and, simultaneously, we successfully monitored early tumor response using Chemical Exchange Saturation Transfer (CEST) MRI. This
[...] Read more.
Treating glioblastoma and monitoring treatment response non-invasively remain challenging. Here, we developed a robust approach using a drug-loaded liposomal hydrogel that is mechanically compatible with the brain, and, simultaneously, we successfully monitored early tumor response using Chemical Exchange Saturation Transfer (CEST) MRI. This CEST-detectable liposomal hydrogel was optimized based on a sustainable drug release and a soft hydrogel for the brain tumor, which is unfavorable for tumor cell proliferation. After injecting the hydrogel next to the tumor, three distinctive CEST contrasts enabled the monitoring of tumor response and drug release longitudinally at 3T. As a result, a continuous tumor volume decrease was observed in the treatment group along with a significant decrease in CEST contrasts relating to the tumor response at 3.5 ppm (Amide Proton Transfer; APT) and at −3.5 ppm (relayed Nuclear Overhauser Effect; rNOE) when compared to the control group (p < 0.05). Interestingly, the molecular change at 3.5 ppm on day 3 (p < 0.05) was found to be prior to the significant decrease in tumor volume on day 5. An APT signal also showed a strong correlation with the number of proliferating cells in the tumors. This demonstrated that APT detected a distinctive decrease in mobile proteins and peptides in tumors before the change in tumor morphology. Moreover, the APT signal showed a regional response to the treatment, associated with proliferating and apoptotic cells, which allowed an in-depth evaluation and prediction of the tumor treatment response. This newly developed liposomal hydrogel allows image-guided brain tumor treatment to address clinical needs using CEST MRI.
Full article
Figure 1
Open AccessReview
Targeted Glioma Therapy—Clinical Trials and Future Directions
Pharmaceutics 2024, 16(1), 100; https://doi.org/10.3390/pharmaceutics16010100 - 11 Jan 2024
Abstract
Glioblastoma multiforme (GBM) is the most common type of glioma, with a median survival of 14.6 months post-diagnosis. Understanding the molecular profile of such tumors allowed the development of specific targeted therapies toward GBM, with a major role attributed to tyrosine kinase receptor
[...] Read more.
Glioblastoma multiforme (GBM) is the most common type of glioma, with a median survival of 14.6 months post-diagnosis. Understanding the molecular profile of such tumors allowed the development of specific targeted therapies toward GBM, with a major role attributed to tyrosine kinase receptor inhibitors and immune checkpoint inhibitors. Targeted therapeutics are drugs that work by specific binding to GBM-specific or overexpressed markers on the tumor cellular surface and therefore contain a recognition moiety linked to a cytotoxic agent, which produces an antiproliferative effect. In this review, we have summarized the available information on the targeted therapeutics used in clinical trials of GBM and summarized current obstacles and advances in targeted therapy concerning specific targets present in GBM tumor cells, outlined efficacy endpoints for major classes of investigational drugs, and discussed promising strategies towards an increase in drug efficacy in GBM.
Full article
(This article belongs to the Special Issue Editorial Board Members’ Collection Series: Targeted Delivery of Anticancer Agents Engaging Cell Specific Mechanisms)
►▼
Show Figures
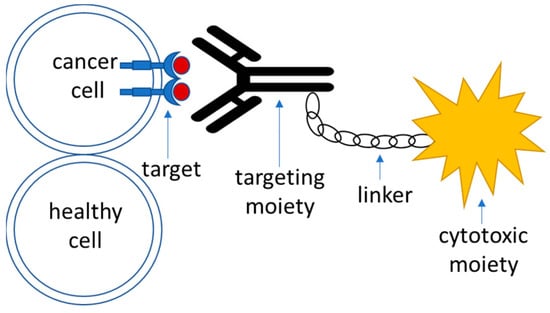
Figure 1
Open AccessArticle
3D-Printed Alginate/Pectin-Based Patches Loaded with Olive Leaf Extracts for Wound Healing Applications: Development, Characterization and In Vitro Evaluation of Biological Properties
by
, , , , , , , , , and
Pharmaceutics 2024, 16(1), 99; https://doi.org/10.3390/pharmaceutics16010099 - 11 Jan 2024
Abstract
Traditional wound dressings may lack suitability for diverse wound types and individual patient requirements. In this context, this study aimed to innovate wound care by developing a 3D-printed patch using alginate and pectin and incorporating Olive Leaf Extract (OLE) as an active ingredient.
[...] Read more.
Traditional wound dressings may lack suitability for diverse wound types and individual patient requirements. In this context, this study aimed to innovate wound care by developing a 3D-printed patch using alginate and pectin and incorporating Olive Leaf Extract (OLE) as an active ingredient. Different polymer-to-plasticizer ratios were systematically examined to formulate a printable ink with optimal viscosity. The resultant film, enriched with OLE, exhibited a substantial polyphenolic content of 13.15 ± 0.41 mg CAE/g, showcasing significant antioxidant and anti-inflammatory properties. Notably, the film demonstrated potent scavenging abilities against DPPH, ABTS, and NO radicals, with IC50 values of 0.66 ± 0.07, 0.47 ± 0.04, and 2.02 ± 0.14 mg/mL, respectively. In vitro release and diffusion studies were carried out and the release profiles revealed an almost complete release of polyphenols from the patch within 48 h. Additionally, the fabricated film exhibited the capacity to enhance cell motility and accelerate wound healing, evidenced by increased collagen I expression in BJ fibroblast cells. Structural assessments affirmed the ability of the patch to absorb exudates and maintain the optimal moisture balance, while biocompatibility studies underscored its suitability for biomedical applications. These compelling findings endorse the potential application of the developed film in advanced wound care, with the prospect of tailoring patches to individual patient needs.
Full article
(This article belongs to the Special Issue Polymer-Based Wound Dressings)
►▼
Show Figures
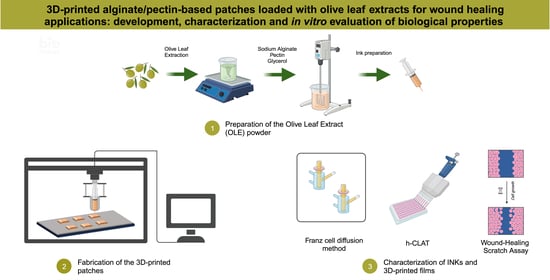
Graphical abstract
Open AccessEditorial
Emerging Trends and Translational Challenges in Drug and Vaccine Delivery
by
and
Pharmaceutics 2024, 16(1), 98; https://doi.org/10.3390/pharmaceutics16010098 - 11 Jan 2024
Abstract
Drug and vaccine delivery have received considerable attention in recent years [...]
Full article
(This article belongs to the Special Issue Emerging Trends and Translational Challenges in Drug and Vaccine Delivery)
Open AccessReview
Towards More Precise Targeting of Inhaled Aerosols to Different Areas of the Respiratory System
Pharmaceutics 2024, 16(1), 97; https://doi.org/10.3390/pharmaceutics16010097 - 10 Jan 2024
Abstract
Pharmaceutical aerosols play a key role in the treatment of lung disorders, but also systemic diseases, due to their ability to target specific areas of the respiratory system (RS). This article focuses on identifying and clarifying the influence of various factors involved in
[...] Read more.
Pharmaceutical aerosols play a key role in the treatment of lung disorders, but also systemic diseases, due to their ability to target specific areas of the respiratory system (RS). This article focuses on identifying and clarifying the influence of various factors involved in the generation of aerosol micro- and nanoparticles on their regional distribution and deposition in the RS. Attention is given to the importance of process parameters during the aerosolization of liquids or powders and the role of aerosol flow dynamics in the RS. The interaction of deposited particles with the fluid environment of the lung is also pointed out as an important step in the mass transfer of the drug to the RS surface. The analysis presented highlights the technical aspects of preparing the precursors to ensure that the properties of the aerosol are suitable for a given therapeutic target. Through an analysis of existing technical limitations, selected strategies aimed at enhancing the effectiveness of targeted aerosol delivery to the RS have been identified and presented. These strategies also include the use of smart inhaling devices and systems with built-in AI algorithms.
Full article
(This article belongs to the Special Issue Drug Delivery Systems for Respiratory Diseases)
►▼
Show Figures
Figure 1
Open AccessArticle
PBPK Modeling Approach to Predict the Behavior of Drugs Cleared by Metabolism in Pregnant Subjects and Fetuses
Pharmaceutics 2024, 16(1), 96; https://doi.org/10.3390/pharmaceutics16010096 - 10 Jan 2024
Abstract
This study aimed to develop a physiologically based pharmacokinetic (PBPK) model that simulates metabolically cleared compounds’ pharmacokinetics (PK) in pregnant subjects and fetuses. This model accounts for the differences in tissue sizes, blood flow rates, enzyme expression levels, plasma protein binding, and other
[...] Read more.
This study aimed to develop a physiologically based pharmacokinetic (PBPK) model that simulates metabolically cleared compounds’ pharmacokinetics (PK) in pregnant subjects and fetuses. This model accounts for the differences in tissue sizes, blood flow rates, enzyme expression levels, plasma protein binding, and other physiological factors affecting the drugs’ PK in both the pregnant woman and the fetus. The PBPKPlus™ module in GastroPlus® was used to model the PK of metoprolol, midazolam, and metronidazole for both non-pregnant and pregnant groups. For each of the three compounds, the model was first developed and validated against PK data in healthy non-pregnant volunteers and then applied to predict the PK in the pregnant groups. The model accurately described the PK in both the non-pregnant and pregnant groups and explained well the differences in the plasma concentration due to pregnancy. When available, the fetal plasma concentration, placenta, and fetal tissue concentrations were also predicted reasonably well at different stages of pregnancy. The work described the use of a PBPK approach for drug development and demonstrates the ability to predict differences in PK in pregnant subjects and fetal exposure for metabolically cleared compounds.
Full article
(This article belongs to the Special Issue Physiologically-Based Pharmacokinetic Modeling in Pregnancy, Lactation, and in Neonates: Achievements, Challenges and Future Directions)
►▼
Show Figures
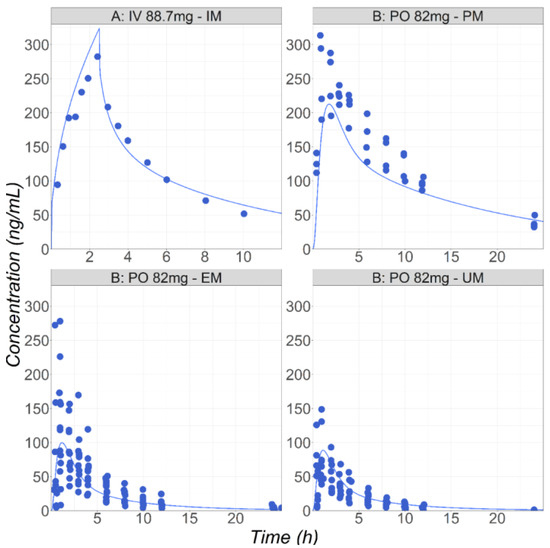
Figure 1
Open AccessArticle
Gemcitabine-Vitamin E Prodrug-Loaded Micelles for Pancreatic Cancer Therapy
by
, , , , , , and
Pharmaceutics 2024, 16(1), 95; https://doi.org/10.3390/pharmaceutics16010095 - 10 Jan 2024
Abstract
Pancreatic cancer (PC) is an aggressive cancer subtype presenting unmet clinical challenges. Conventional chemotherapy, which includes antimetabolite gemcitabine (GEM), is seriously undermined by a short half-life, its lack of targeting ability, and systemic toxicity. GEM incorporation in self-assembled nanosystems is still underexplored due
[...] Read more.
Pancreatic cancer (PC) is an aggressive cancer subtype presenting unmet clinical challenges. Conventional chemotherapy, which includes antimetabolite gemcitabine (GEM), is seriously undermined by a short half-life, its lack of targeting ability, and systemic toxicity. GEM incorporation in self-assembled nanosystems is still underexplored due to GEM’s hydrophilicity which hinders efficient encapsulation. We hypothesized that vitamin E succinate–GEM prodrug (VES-GEM conjugate) combines hydrophobicity and multifunctionalities that can facilitate the development of Pluronic® F68 and Pluronic® F127 micelle-based nanocarriers, improving the therapeutic potential of GEM. Pluronic® F68/VES-GEM and Pluronic® F127/VES-GEM micelles covering a wide range of molar ratios were prepared by solvent evaporation applying different purification methods, and characterized regarding size, charge, polydispersity index, morphology, and encapsulation. Moreover, the effect of sonication and ultrasonication and the influence of a co-surfactant were explored together with drug release, stability, blood compatibility, efficacy against tumour cells, and cell uptake. The VES-GEM conjugate-loaded micelles showed acceptable size and high encapsulation efficiency (>95%) following an excipient reduction rationale. Pluronic® F127/VES-GEM micelles evidenced a superior VES-GEM release profile (cumulative release > 50%, pH = 7.4), stability, cell growth inhibition (<50% cell viability for 100 µM VES-GEM), blood compatibility, and extensive cell internalization, and therefore represent a promising approach to leveraging the efficacy and safety of GEM for PC-targeted therapies.
Full article
(This article belongs to the Special Issue Advances of Colloidal Systems in Drug Delivery and Therapeutic Application, 2nd Edition)
►▼
Show Figures
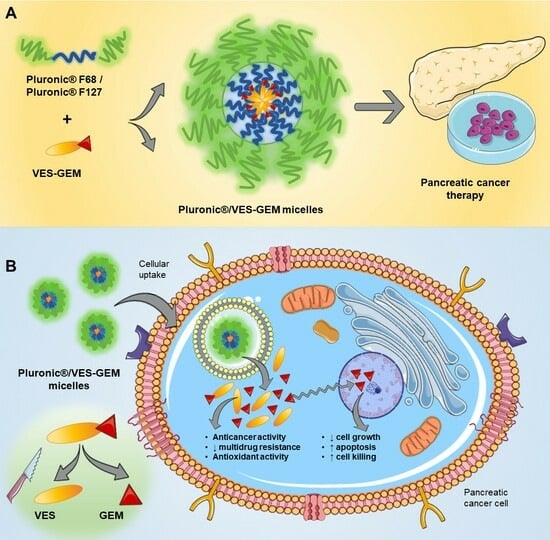
Graphical abstract
Open AccessArticle
Pharmacokinetic Interpretation of Applying Local Drug Delivery System for the Treatment of Deep Surgical Site Infection in the Spine
by
and
Pharmaceutics 2024, 16(1), 94; https://doi.org/10.3390/pharmaceutics16010094 - 10 Jan 2024
Abstract
Surgical site infections (SSIs) after spinal surgery present significant challenges, including poor antibiotic penetration and biofilm formation on implants, leading to frequent treatment failures. Polymethylmethacrylate (PMMA) is widely used for localized drug delivery in bone infections, yet quantifying individual drug release kinetics is
[...] Read more.
Surgical site infections (SSIs) after spinal surgery present significant challenges, including poor antibiotic penetration and biofilm formation on implants, leading to frequent treatment failures. Polymethylmethacrylate (PMMA) is widely used for localized drug delivery in bone infections, yet quantifying individual drug release kinetics is often impractical. This retrospective study analyzed 23 cases of deep SSIs (DSSIs) following spinal surgery treated with antibiotic-loaded PMMA. A mathematical model estimated personalized drug release kinetics from PMMA, considering disease types, pathogens, and various antibiotics. The study found that vancomycin (VAN), ceftriaxone (CRO), and ceftazidime (CAZ) reached peak concentrations of 15.43%, 15.42%, and 15.41%, respectively, within the first two days, which was followed by a lag phase (4.91–4.92%) on days 2–3. On days 5–7, concentrations stabilized, with CRO at 3.22% and CAZ/VAN between 3.63% and 3.65%, averaging 75.4 µg/cm2. Key factors influencing release kinetics include solubility, diffusivity, porosity, tortuosity, and bead diameter. Notably, a patient with a low glomerular filtration rate (ASA IV) was successfully treated with a shortened 9-day intravenous VAN regimen, avoiding systemic complications. This study affirms the effectiveness of local drug delivery systems (DDS) in treating DSSIs and underscores the value of mathematical modeling in determining drug release kinetics. Further research is essential to optimize release rates and durations and to mitigate risks of burst release and tissue toxicity.
Full article
(This article belongs to the Special Issue Drug Delivery Systems for Bone Targeted Therapeutics: Current Approach and Future Perspectives)
►▼
Show Figures
Figure 1

Journal Menu
► ▼ Journal Menu-
- Pharmaceutics Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
9 January 2024
Meet Us at the 14th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology, 18–21 March 2024, Vienna, Austria
Meet Us at the 14th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology, 18–21 March 2024, Vienna, Austria

2 January 2024
MDPI Insights: The CEO's Letter #7 - Nobel Laureates Entrust MDPI with Their Research
MDPI Insights: The CEO's Letter #7 - Nobel Laureates Entrust MDPI with Their Research
Topics
Topic in
IJMS, Materials, Nanomaterials, Pharmaceutics, Polymers
Nanomaterials and Polymers in Controlled Drug Delivery
Topic Editors: Ziyad S. Haidar, Prabir PatraDeadline: 31 January 2024
Topic in
Biomedicines, Cancers, JCM, Nanomaterials, Pharmaceutics
Application of Nanomaterials and Nanobiotechnology in Cancer
Topic Editors: Ayan Kumar Barui, Susheel Kumar NethiDeadline: 31 March 2024
Topic in
Pharmaceuticals, Pharmaceutics, Antioxidants, Nanomaterials, JFB, Cosmetics
New Challenges in the Cosmetics and Medical Device Industry
Topic Editors: Ana Catarina Silva, Hugo Almeida, Ana BarrosDeadline: 30 April 2024
Topic in
Bioengineering, Biomolecules, Cancers, Diseases, Nanomaterials, Pharmaceutics
Dynamic Nano-Biomaterials in Tissue Regeneration and Cancer Therapies
Topic Editors: Ramar Thangam, Heemin Kang, Bibin G. Anand, Ramachandran Vijayan, Venugopal KrishnanDeadline: 15 May 2024

Conferences
Special Issues
Special Issue in
Pharmaceutics
Machine Learning for Dosage Forms Developments
Guest Editor: Svetlana IbrićDeadline: 20 January 2024
Special Issue in
Pharmaceutics
Organic-Inorganic Nanocomposites as Delivery Systems of Therapeutic Agents for Regenerative Medicine
Guest Editors: Kai Zheng, Annabel BraemDeadline: 31 January 2024
Special Issue in
Pharmaceutics
Microenvironment-Responsive Delivery Systems
Guest Editor: Yiran ZhengDeadline: 10 February 2024
Special Issue in
Pharmaceutics
Continuous Pharmaceutical Manufacturing, Volume II
Guest Editors: Ossi Korhonen, Tuomas ErvastiDeadline: 20 February 2024
Topical Collections
Topical Collection in
Pharmaceutics
Feature Papers in Pharmaceutical Technology
Collection Editor: Thierry Vandamme
Topical Collection in
Pharmaceutics
Advanced Pharmaceutical Science and Technology in Korea
Collection Editors: Hyo-Kyung Han, Beom-Jin Lee
Topical Collection in
Pharmaceutics
Advanced Pharmaceutical Science and Technology in Estonia
Collection Editors: Karin Kogermann, Jana Lass
Topical Collection in
Pharmaceutics
Women in Pharmaceutics
Collection Editors: Donatella Paolino, Cinzia Anna Ventura












