-
 Orogenital Human Papillomavirus Infection and Vaccines: A Survey of High- and Low-Risk Genotypes Not Included in Vaccines
Orogenital Human Papillomavirus Infection and Vaccines: A Survey of High- and Low-Risk Genotypes Not Included in Vaccines -
 Efficiency Comparative Approach of Plant-Produced Monoclonal Antibodies against Rabies Virus Infection
Efficiency Comparative Approach of Plant-Produced Monoclonal Antibodies against Rabies Virus Infection -
 A Comprehensive Review on Cancer Vaccines and Vaccine Strategies in Hepatocellular Carcinoma
A Comprehensive Review on Cancer Vaccines and Vaccine Strategies in Hepatocellular Carcinoma
Journal Description
Vaccines
Vaccines
is an international, peer-reviewed, open access journal published monthly online by MDPI. The American Society for Virology (ASV) is affiliated with Vaccines and their members receive a discount on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q1 (Immunology) / CiteScore - Q1 (Pharmacology (medical))
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 19.2 days after submission; acceptance to publication is undertaken in 2.9 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
7.8 (2022);
5-Year Impact Factor:
7.4 (2022)
Latest Articles
A Potential Association between Abdominal Obesity and the Efficacy of Humoral Immunity Induced by COVID-19 and by the AZD1222, Convidecia, BNT162b2, Sputnik V, and CoronaVac Vaccines
Vaccines 2024, 12(1), 88; https://doi.org/10.3390/vaccines12010088 - 15 Jan 2024
Abstract
Abdominal obesity is highly prevalent in Mexico and has a poor prognosis in terms of the severity of coronavirus disease (COVID-19) and low levels of antibodies induced by infection and vaccination. We evaluated the humoral immune response induced by COVID-19 and five different
[...] Read more.
Abdominal obesity is highly prevalent in Mexico and has a poor prognosis in terms of the severity of coronavirus disease (COVID-19) and low levels of antibodies induced by infection and vaccination. We evaluated the humoral immune response induced by COVID-19 and five different vaccination schedules in Mexican individuals with abdominal obesity and the effects of other variables. This prospective longitudinal cohort study included 2084 samples from 389 participants. The levels of anti-S1/S2 and anti-RBD IgG antibodies were measured at various time points after vaccination. A high prevalence of hospitalization and oxygen use was observed in individuals with abdominal obesity (AO) who had COVID-19 before vaccination; however, they also had high levels of anti-S1/S2 and anti-RBD-neutralizing IgG antibodies. The same was true for vaccination-induced antibody levels. However, their longevity was low. Interestingly, we did not observe significant differences in vaccine reactogenicity between abdominally obese and abdominally non-obese groups. Finally, individuals with a higher body mass index, older age, and previous COVID-19 had higher levels of antibodies induced by COVID-19 and vaccination. Therefore, it is important to evaluate other immunological and inflammatory factors to better understand the pathogenesis of COVID-19 in the presence of risk factors and to propose effective vaccination schedules for vulnerable populations.
Full article
(This article belongs to the Special Issue Safety, Efficacy and Optimization of the COVID-19 Vaccines)
►
Show Figures
Open AccessReview
Development of Vaccines against Emerging Mosquito-Vectored Arbovirus Infections
by
and
Vaccines 2024, 12(1), 87; https://doi.org/10.3390/vaccines12010087 - 15 Jan 2024
Abstract
Among emergent climate-sensitive infectious diseases, some mosquito-vectored arbovirus infections have epidemiological, social, and economic effects. Dengue virus (DENV), West Nile virus (WNV), and Chikungunya virus (CHIKV) disease, previously common only in the tropics, currently pose a major risk to global health and are
[...] Read more.
Among emergent climate-sensitive infectious diseases, some mosquito-vectored arbovirus infections have epidemiological, social, and economic effects. Dengue virus (DENV), West Nile virus (WNV), and Chikungunya virus (CHIKV) disease, previously common only in the tropics, currently pose a major risk to global health and are expected to expand dramatically in the near future if adequate containment measures are not implemented. The lack of safe and effective vaccines is critical as it seems likely that emerging mosquito-vectored arbovirus infections will be con-trolled only when effective and safe vaccines against each of these infections become available. This paper discusses the clinical characteristics of DENV, WNV, and CHIKV infections and the state of development of vaccines against these viruses. An ideal vaccine should be able to evoke with a single administration a prompt activation of B and T cells, adequate concentrations of protecting/neutralizing antibodies, and the creation of a strong immune memory capable of triggering an effective secondary antibody response after new infection with a wild-type and/or mutated infectious agent. Moreover, the vaccine should be well tolerated, safe, easily administrated, cost-effective, and widely available throughout the world. However, the development of vaccines against emerging mosquito-vectored arbovirus diseases is far from being satisfactory, and it seems likely that it will take many years before effective and safe vaccines for all these infections are made available worldwide.
Full article
(This article belongs to the Special Issue Vaccine Development for Arboviruses)
Open AccessArticle
Analysis of CVC1302-Mediated Enhancement of Monocyte Recruitment in Inducing Immune Responses
by
, , , , , , , , , and
Vaccines 2024, 12(1), 86; https://doi.org/10.3390/vaccines12010086 - 15 Jan 2024
Abstract
►▼
Show Figures
Monocytes (Mos) are believed to play important roles during the generation of immune response. In our previous study, CVC1302, a complex of PRRs agonists, was demonstrated to recruit Mo into lymph nodes (LNs) in order to present antigen and secret chemokines (CXCL9 and
[...] Read more.
Monocytes (Mos) are believed to play important roles during the generation of immune response. In our previous study, CVC1302, a complex of PRRs agonists, was demonstrated to recruit Mo into lymph nodes (LNs) in order to present antigen and secret chemokines (CXCL9 and CXCL10), which attracted antigen-specific CD4+ T cells. As it is known that Mos in mice are divided into two main Mo subsets (Ly6C+ Mo and Ly6C− Mo), we aimed to clarify the CVC1302-recruiting Mo subset and functions in the establishment of immunity. In this study, we found that CVC1302 attracted both Ly6C+ Mo and Ly6C− Mo into draining LNs, which infiltrated from different origins, injection muscles and high endothelial venule (HEV), respectively. We also found that the numbers of OVA+ Ly6C+ Mo in the draining LNs were significantly higher compared with OVA+ Ly6C− Mo. However, the levels of CXCL9 and CXCL10 produced by Ly6C− Mo were significantly higher than Ly6C+ Mo, which plays important roles in attracting antigen-specific CD4+ T cells. Under the analysis of their functions in initiating immune responses, we found that the ability of the Ly6C+ monocyte was mainly capturing and presenting antigens, otherwise; the ability of the Ly6C− monocyte was mainly secreting CXCL9 and CXCL10, which attracted antigen-specific CD4+ T cells through CXCR3. These results will provide new insights into the development of new immunopotentiators and vaccines.
Full article
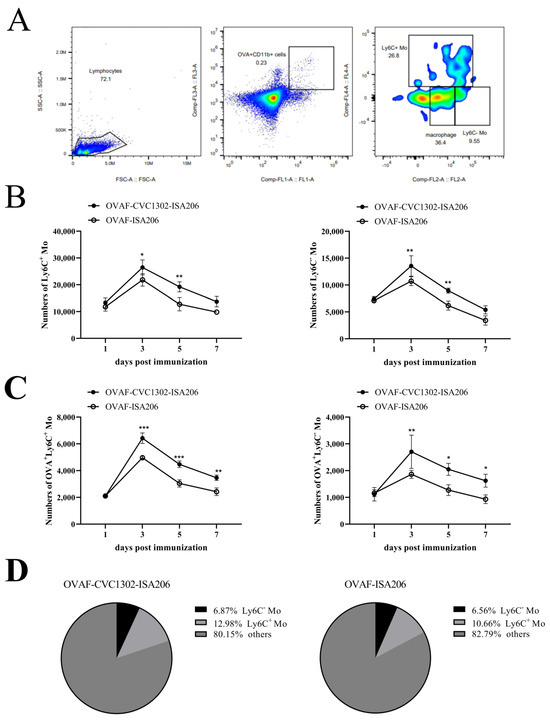
Figure 1
Open AccessArticle
The Risk of Herpes Zoster Events in Patients with Spondyloarthritis and the Effect of BNT162b2 mRNA COVID-19 Vaccine
by
, , , , , , and
Vaccines 2024, 12(1), 85; https://doi.org/10.3390/vaccines12010085 - 15 Jan 2024
Abstract
The data on the risk of herpes zoster (HZ) in spondyloarthropathy (SpA) patients are sparse, especially regarding its association with the novel mRNA COVID-19 vaccines and immunosuppressants. We aimed to evaluate whether SpA diagnosis and/or immunosuppressant use affect HZ risk and the influence
[...] Read more.
The data on the risk of herpes zoster (HZ) in spondyloarthropathy (SpA) patients are sparse, especially regarding its association with the novel mRNA COVID-19 vaccines and immunosuppressants. We aimed to evaluate whether SpA diagnosis and/or immunosuppressant use affect HZ risk and the influence of mRNA COVID-19 vaccination. We assessed the association between SpA (psoriatic arthritis (PsA) and ankylosing spondylitis (AS)) diagnoses and HZ in a large population database with patients matched by age and sex to controls. We also assessed the association between the COVID-19 vaccine and new-onset HZ using two nested case–control studies, identifying all new HZ cases diagnosed from 1 January–31 December 2021 within the SpA and general population cohorts, matched randomly by sex, age and HZ index date to controls without HZ. Exposure to mRNA COVID-19 vaccination was ascertained in the 6 weeks prior to the index date both in cases and controls. In our results, the incidence rate of HZ was higher in PsA patients vs. the general population, at 1.03 vs. 0.64 per 100 person-years, respectively (adjusted HR = 1.55; 95%CI, 1.19–2.02). Within the SpA group, Jak-I treatment was associated with a higher risk of developing new-onset HZ (adjusted OR = 3.79; 1.15–12.5). Multivariable conditional logistic regression models we used showed no association between COVID-19 vaccination and new-onset HZ among the SpA patients (OR = 1.46; 0.68–3.14).
Full article
(This article belongs to the Special Issue Safety and Effectiveness of COVID-19 Vaccines on COVID-19 Infection and Its Long-Term Consequences)
Open AccessArticle
Determinants of Human Papillomavirus Vaccine Acceptance among Caregivers in Nigeria: A Fogg Behavior Model-Based Approach
Vaccines 2024, 12(1), 84; https://doi.org/10.3390/vaccines12010084 - 13 Jan 2024
Abstract
Human papillomavirus (HPV) vaccine uptake among adolescent girls is critical to reducing the burden of HPV-related cancers in Nigeria. This study assesses the factors influencing caregivers’ acceptance of HPV vaccination for their charges, using the Fogg Behavior Model (FBM) as a theoretical framework.
[...] Read more.
Human papillomavirus (HPV) vaccine uptake among adolescent girls is critical to reducing the burden of HPV-related cancers in Nigeria. This study assesses the factors influencing caregivers’ acceptance of HPV vaccination for their charges, using the Fogg Behavior Model (FBM) as a theoretical framework. We analyzed cross-sectional data from 1429 caregivers of girls aged 9–17 in six Nigerian states, using a survey instrument based on the FBM. Participants were recruited via Facebook and Instagram advertisements and interviewed through Facebook Messenger in August and September 2023. The study received ethical clearance from Nigeria’s National Health Research Ethics Committee. We applied bivariate and multivariate analyses to assess the relationships between the caregiver’s perception of how likely their adolescent girl was to get vaccinated in the next 12 months and motivation, ability, social factors (such as discussions with family and friends), injunctive norms, previous COVID-19 vaccination, and respondents’ sociodemographic characteristics. Adjusted odds ratios derived from logistic regression analyses revealed that caregivers’ motivation and ability, as well as social factors, were significantly associated with their perception that the adolescent girl in their care would get vaccinated within the next 12 months. Our findings suggest that behavioral interventions tailored to enhance motivation, ability, and social support among caregivers could significantly increase HPV vaccine uptake among adolescent girls in Nigeria.
Full article
(This article belongs to the Special Issue HPV Vaccination: Current Situation and Future Goals)
Open AccessArticle
Burden and Impact of Reactogenicity among Adults Receiving COVID-19 Vaccines in the United States and Canada: Results from a Prospective Observational Study
by
, , , , , , , , and
Vaccines 2024, 12(1), 83; https://doi.org/10.3390/vaccines12010083 - 13 Jan 2024
Abstract
►▼
Show Figures
As SARS-CoV-2 variants continue to emerge, vaccination remains a critical tool to reduce the COVID-19 burden. Vaccine reactogenicity and the impact on work productivity/daily activities are recognized as contributing factors to vaccine hesitancy. To encourage continued COVID-19 vaccination, a more complete understanding of
[...] Read more.
As SARS-CoV-2 variants continue to emerge, vaccination remains a critical tool to reduce the COVID-19 burden. Vaccine reactogenicity and the impact on work productivity/daily activities are recognized as contributing factors to vaccine hesitancy. To encourage continued COVID-19 vaccination, a more complete understanding of the differences in reactogenicity and impairment due to vaccine-related side effects across currently available vaccines is necessary. The 2019nCoV-406 study (n = 1367) was a prospective observational study of reactogenicity and associated impairments in adults in the United States and Canada who received an approved/authorized COVID-19 vaccine. Compared with recipients of mRNA COVID-19 booster vaccines, a smaller percentage of NVX-CoV2373 booster recipients reported local and systemic reactogenicity. This study’s primary endpoint (percentage of participants with ≥50% overall work impairment on ≥1 of the 6 days post-vaccination period) did not show significant differences. However, the data suggest that NVX-CoV2373 booster recipients trended toward being less impaired overall than recipients of an mRNA booster; further research is needed to confirm this observed trend. The results of this real-world study suggest that NVX-CoV2373 may be a beneficial vaccine option with limited impact on non-work activities, in part due to the few reactogenicity events after vaccination.
Full article
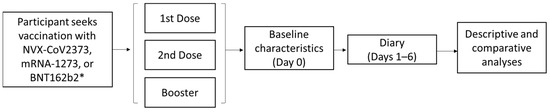
Figure 1
Open AccessReview
Vaccinations in Selected Immune-Related Diseases Treated with Biological Drugs and JAK Inhibitors—Literature Review and Statement of Experts from Polish Dermatological Society
Vaccines 2024, 12(1), 82; https://doi.org/10.3390/vaccines12010082 - 13 Jan 2024
Abstract
The growing use of biological drugs in immune-mediated chronic diseases has undoubtedly revolutionized their treatment. Yet, the topic of vaccinations in this group of patients still raises many concerns and implies many therapeutic problems that require discussion and standardization of management. The aim
[...] Read more.
The growing use of biological drugs in immune-mediated chronic diseases has undoubtedly revolutionized their treatment. Yet, the topic of vaccinations in this group of patients still raises many concerns and implies many therapeutic problems that require discussion and standardization of management. The aim of this literature review is to present current knowledge regarding safety and efficacy of vaccinations in dermatological and rheumatological patients treated with biological drugs and JAK inhibitors. Additionally, this article provides recommendation from experts of the Polish Dermatological Society about proper use of vaccinations during therapy with biologics. Generally, all live attenuated vaccines are contraindicated during immunosuppressive/immunomodulatory therapy. If there is need, they should be administered long enough prior to the therapy or after cessation. Yet, inactivated vaccines mostly can be safely used, but the problem in this case is the effectiveness of the vaccination. Most studies report that the immune response in patients on biologics after administration of different inactivated vaccines is similar to or even better than in the control group. Thus, the importance of vaccination among patients on biologics must be emphasized to reduce omissions and the fear of possible side effects or insufficient post-vaccination response.
Full article
Open AccessArticle
Mathematical Optimization Strategy for Effectiveness Profile Estimation in Two-Dose Vaccines and Its Use in Designing Improved Vaccination Strategies Focused on Pandemic Containment
Vaccines 2024, 12(1), 81; https://doi.org/10.3390/vaccines12010081 - 12 Jan 2024
Abstract
Since late 2019, most efforts to control the COVID-19 pandemic have focused on developing vaccines. By mid-2020, some vaccines fulfilled international regulations for their application. However, these vaccines have shown a decline in effectiveness several weeks after the last dose, highlighting the need
[...] Read more.
Since late 2019, most efforts to control the COVID-19 pandemic have focused on developing vaccines. By mid-2020, some vaccines fulfilled international regulations for their application. However, these vaccines have shown a decline in effectiveness several weeks after the last dose, highlighting the need to optimize vaccine administration due to supply chain limitations. While methods exist to prioritize population groups for vaccination, there is a lack of research on how to optimally define the time between doses when two-dose vaccines are administrated to such groups. Under such conditions, modeling the real effect of each vaccine on the population is critical. Even though several efforts have been made to characterize vaccine effectiveness profiles, none of these initiatives enable characterization of the individual effect of each dose. Thus, this paper presents a novel methodology for estimating the vaccine effectiveness profile. It addresses the vaccine characterization problem by considering a deconvolution of relevant data profiles, treating them as an optimization process. The results of this approach enabled the independent estimation of the effectiveness profiles for the first and second vaccine doses and their use to find sweet spots for designing efficient vaccination strategies. Our methodology can enable a more effective and efficient contemporary response against the COVID-19 pandemic, as well as for any other disease in the future.
Full article
(This article belongs to the Special Issue Efficacy, Immunogenicity and Safety of COVID-19 Vaccines and COVID-19 Vaccination Strategies)
►▼
Show Figures
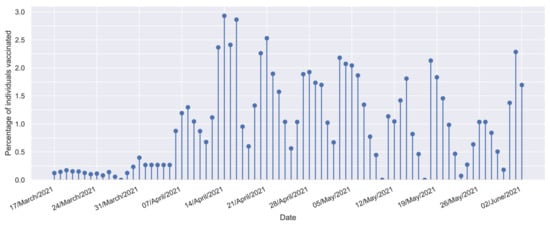
Figure 1
Open AccessArticle
Exploring Perceptions and Practices Regarding Adult Vaccination against Seasonal Influenza, Tetanus, Pneumococcal Disease, Herpes Zoster and COVID-19: A Mixed-Methods Study in Greece
by
, , , , , and
Vaccines 2024, 12(1), 80; https://doi.org/10.3390/vaccines12010080 - 12 Jan 2024
Abstract
We aimed to document vaccination coverage for five vaccines, predictors of each vaccine’s uptake and attitudes regarding adult vaccination. Adults visiting four pharmacies were randomly invited to participate during summer 2022. Among 395 participants (mean age 51.2 years, range 19–96), vaccination rates were
[...] Read more.
We aimed to document vaccination coverage for five vaccines, predictors of each vaccine’s uptake and attitudes regarding adult vaccination. Adults visiting four pharmacies were randomly invited to participate during summer 2022. Among 395 participants (mean age 51.2 years, range 19–96), vaccination rates were 78.1% for influenza and 25.8% for herpes zoster (≥60 years old), 64.3% for pneumococcal disease (≥65 years old), 33.1% for tetanus, while 11.4% had received two and 74.8% ≥3 COVID-19 vaccine doses. Half of participants (50.1%) voiced some degree of hesitancy, and 1.3% were refusers. The strongest predictor of each vaccine’s uptake was doctor’s recommendation (OR range 11.33–37.66, p < 0.001) and pharmacist’s recommendation (4.01–19.52, p < 0.05), except for the COVID-19 vaccine, where the Attitude Towards Adult VACcination (ATAVAC) value of adult vaccination subscale’s score was the only predictor (OR: 5.75, p < 0.001). Regarding insufficient coverage, thematic content analysis revealed seven main themes. Insufficient knowledge, the absence of health professionals’ recommendation, perception of low susceptibility to disease, negligence and dispute of vaccine effectiveness were universal themes, whereas safety concerns and distrust in authorities were reported solely for COVID-19 vaccination. Designing public interventions aiming to increase trust in adult vaccination is essential in the aftermath of the COVID-19 pandemic. Health professionals’ role in recommending strongly adult vaccination is crucial.
Full article
(This article belongs to the Special Issue Promoting Vaccination in the Post-COVID-19 Era)
Open AccessEditorial
The Impact of COVID-19 and Non-COVID-19 Vaccinations in Special Populations
Vaccines 2024, 12(1), 79; https://doi.org/10.3390/vaccines12010079 - 12 Jan 2024
Abstract
Vaccination to prevent human infection is a key driver for reducing morbidity and mortality [...]
Full article
(This article belongs to the Special Issue Impact of COVID-19 and Non-COVID-19 Vaccination in Special Populations)
Open AccessArticle
The Development of a Rabies Virus-Vectored Vaccine against Borrelia burgdorferi, Targeting BBI39
by
, , , , and
Vaccines 2024, 12(1), 78; https://doi.org/10.3390/vaccines12010078 - 12 Jan 2024
Abstract
Lyme disease (LD) is the most common tick-borne illness in the United States (U.S.), Europe, and Asia. Borrelia burgdorferi, a spirochete bacterium transmitted by the tick vector Ixodes scapularis, causes LD in the U.S. If untreated, Lyme arthritis, heart block, and
[...] Read more.
Lyme disease (LD) is the most common tick-borne illness in the United States (U.S.), Europe, and Asia. Borrelia burgdorferi, a spirochete bacterium transmitted by the tick vector Ixodes scapularis, causes LD in the U.S. If untreated, Lyme arthritis, heart block, and meningitis can occur. Given the absence of a human Lyme disease vaccine, we developed a vaccine using the rabies virus (RABV) vaccine vector BNSP333 and an outer surface borrelial protein, BBI39. BBI39 was previously utilized as a recombinant protein vaccine and was protective in challenge experiments; therefore, we decided to utilize this protective antigen in a rabies virus-vectored vaccine against Borrelia burgdorferi. To incorporate BBI39 into the RABV virion, we generated a chimeric BBI39 antigen, BBI39RVG, by fusing BBI39 with the final amino acids of the RABV glycoprotein by molecular cloning and viral recovery with reverse transcription genetics. Here, we have demonstrated that the BBI39RVG antigen was incorporated into the RABV virion via immunofluorescence and Western blot analysis. Mice vaccinated with our BPL inactivated RABV-BBI39RVG (BNSP333-BBI39RVG) vaccine induced high amounts of BBI39-specific antibodies, which were maintained long-term, up to eight months post-vaccination. The BBI39 antibodies neutralized Borrelia in vaccinated mice when challenged with Borrelia burgdorferi by either syringe injection or infected ticks and they reduced the Lyme disease pathology of arthritis in infected mouse joints. Overall, the RABV-based LD vaccine induced more and longer-term antibodies compared to the recombinant protein vaccine. This resulted in lower borrelial RNA in RABV-based vaccinated mice compared to recombinant protein vaccinated mice. The results of this study indicate the successful use of BBI39 as a vaccine antigen and RABV as a vaccine vector for LD.
Full article
(This article belongs to the Section Attenuated/Inactivated/Live and Vectored Vaccines)
►▼
Show Figures
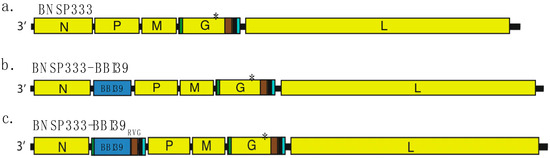
Figure 1
Open AccessArticle
A Mixture of T-Cell Epitope Peptides Derived from Human Respiratory Syncytial Virus F Protein Conferred Protection in DR1-TCR Tg Mice
by
, , , , , , , , and
Vaccines 2024, 12(1), 77; https://doi.org/10.3390/vaccines12010077 - 12 Jan 2024
Abstract
Human respiratory syncytial virus (HRSV) poses a significant disease burden on global health. To date, two vaccines that primarily induce humoral immunity to prevent HRSV infection have been approved, whereas vaccines that primarily induce T-cell immunity have not yet been well-represented. To address
[...] Read more.
Human respiratory syncytial virus (HRSV) poses a significant disease burden on global health. To date, two vaccines that primarily induce humoral immunity to prevent HRSV infection have been approved, whereas vaccines that primarily induce T-cell immunity have not yet been well-represented. To address this gap, 25 predicted T-cell epitope peptides derived from the HRSV fusion protein with high human leukocyte antigen (HLA) binding potential were synthesized, and their ability to be recognized by PBMC from previously infected HRSV cases was assessed using an ELISpot assay. Finally, nine T-cell epitope peptides were selected, each of which was recognized by at least 20% of different donors’ PBMC as potential vaccine candidates to prevent HRSV infection. The protective efficacy of F-9PV, a combination of nine peptides along with CpG-ODN and aluminum phosphate (Al) adjuvants, was validated in both HLA-humanized mice (DR1-TCR transgenic mice, Tg mice) and wild-type (WT) mice. The results show that F-9PV significantly enhanced protection against viral challenge as evidenced by reductions in viral load and pathological lesions in mice lungs. In addition, F-9PV elicits robust Th1-biased response, thereby mitigating the potential safety risk of Th2-induced respiratory disease during HRSV infection. Compared to WT mice, the F-9PV mice exhibited superior protection and immunogenicity in Tg mice, underscoring the specificity for human HLA. Overall, our results demonstrate that T-cell epitope peptides provide protection against HRSV infection in animal models even in the absence of neutralizing antibodies, indicating the feasibility of developing an HRSV T-cell epitope peptide-based vaccine.
Full article
(This article belongs to the Special Issue Bacterial and Viral Immunity and Vaccination)
►▼
Show Figures
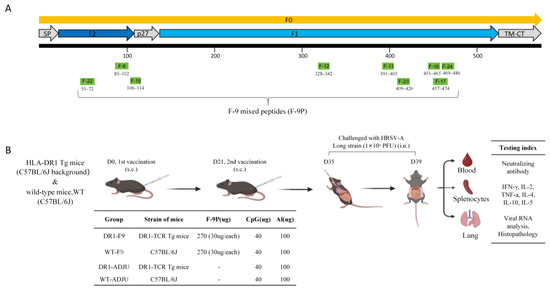
Figure 1
Open AccessArticle
Humoral Response and Safety after a Fourth Dose of the SARS-CoV-2 BNT162b2 Vaccine in Cancer Patients Undergoing Active Treatment—Results of a Prospective Observational Study
Vaccines 2024, 12(1), 76; https://doi.org/10.3390/vaccines12010076 - 12 Jan 2024
Abstract
Only a few studies have been carried out on the efficacy and safety of a fourth dose of the COVID-19 vaccine in patients with cancer. In this prospective observational study, we aimed to assess the serological response and safety of the fourth booster
[...] Read more.
Only a few studies have been carried out on the efficacy and safety of a fourth dose of the COVID-19 vaccine in patients with cancer. In this prospective observational study, we aimed to assess the serological response and safety of the fourth booster shot of the BNT162b2 vaccine in 79 cancer patients, vaccinated between 1 March and 25 August 2022, under systemic anticancer therapy. The primary endpoint was to assess the increase in the anti-SARS-CoV-2 antibodies; secondary endpoints were the vaccine safety and side effects. Consequently, 40 patients (50.63%) revealed the maximum detection values in their IgG titers before the fourth dose of the vaccine, while 39 patients (49.37%) did not. Primary endpoint: Of 39 patients, 36 (92.31%) showed a significant increase in the anti-SARS-CoV-2 IgG titers, and 32 of them (82.05%) reached the maximum titration values. Secondary endpoints: The most common adverse events were mild in severity and included injection site pain, erythema and tiredness. The majority of the adverse reactions reported were grade 1 and no grade 3 and 4 reactions were detected. Our data provide evidence that a fourth dose of the BNT162b2 anti-SARS-CoV-2 vaccine is effective and safe in patients with solid tumors in active anticancer treatment.
Full article
(This article belongs to the Special Issue Safety, Efficacy and Optimization of the COVID-19 Vaccines)
Open AccessArticle
Coverage with the First Dose of Human Papillomavirus Vaccination among Females Aged 9–50 Years in Shenzhen, China: A Surveillance Based on Administrative Health Records in 2023
by
, , , , , , , , , and
Vaccines 2024, 12(1), 75; https://doi.org/10.3390/vaccines12010075 - 12 Jan 2024
Abstract
China started to offer human papillomavirus (HPV) vaccines to females aged 9–45 years in 2016. However, there was a lack of reports about HPV vaccination coverage in a representative sample of females in China. Therefore, this study aimed to examine the current HPV
[...] Read more.
China started to offer human papillomavirus (HPV) vaccines to females aged 9–45 years in 2016. However, there was a lack of reports about HPV vaccination coverage in a representative sample of females in China. Therefore, this study aimed to examine the current HPV coverage and associated factors among females aged 9–50 years in Shenzhen, China, based on administrative health records kept by community health centers. A multistage random sampling approach was used. The research team randomly selected 18 community health centers in Shenzhen, and 3118 health records of females aged 9–50 years were then randomly selected from these health centers. Among all participants, 18.7% received at least one dose of HPV vaccination. The highest coverage was observed among females aged 18–26 years (23.4%), followed by those aged 27–35 years (22.0%) and 36–45 years (20.2%). Such coverage was very low among females aged 9–17 years (4.6%) and those aged 46–50 years (3.2%). Among females aged 18 years or above, higher education level, having a family doctor, and permanent residency in Shenzhen were associated with higher HPV vaccination coverage, while older age and being married/divorced were negatively associated with coverage. The HPV vaccination coverage in Shenzhen was 18.7% and there is a strong need for improvement.
Full article
(This article belongs to the Section Human Vaccines and Public Health)
Open AccessArticle
Going Forward: Potential Impact of Protein-Based COVID-19 Vaccination Coverage on Population Outcomes and Costs in the United States
by
, , , , and
Vaccines 2024, 12(1), 74; https://doi.org/10.3390/vaccines12010074 - 12 Jan 2024
Abstract
Policymakers in the United States (US) recommend coronavirus disease 2019 (COVID-19) vaccination with a monovalent 2023–2024 vaccine formulation based on the Omicron XBB.1.5 variant. We estimated the potential US population-level health and economic impacts of increased COVID-19 vaccine coverage that might be expected
[...] Read more.
Policymakers in the United States (US) recommend coronavirus disease 2019 (COVID-19) vaccination with a monovalent 2023–2024 vaccine formulation based on the Omicron XBB.1.5 variant. We estimated the potential US population-level health and economic impacts of increased COVID-19 vaccine coverage that might be expected with the availability of a protein-based vaccine with simpler storage requirements in addition to messenger ribonucleic acid (mRNA) vaccines. A Markov model was developed to estimate 1-year COVID-19-related costs, cases, hospitalizations, and deaths with and without the availability of a protein-based vaccine option. The model population was stratified by age and risk status. Model inputs were sourced from published literature or derived from publicly available data. Our model estimated that a five-percentage-point increase in coverage due to the availability of a protein-based vaccine option would prevent over 500,000 cases, 66,000 hospitalizations, and 3000 COVID-19-related deaths. These clinical outcomes translated to 42,000 quality-adjusted life years (QALYs) gained and an incremental cost–effectiveness ratio of USD 16,141/QALY from a third-party payer perspective. In sensitivity analyses, outcomes were most sensitive to COVID-19 incidence and severity across age groups. The availability of a protein-based vaccine option in the US could reduce hospitalizations and deaths and is predicted to be cost-effective.
Full article
(This article belongs to the Section COVID-19 Vaccines and Vaccination)
►▼
Show Figures
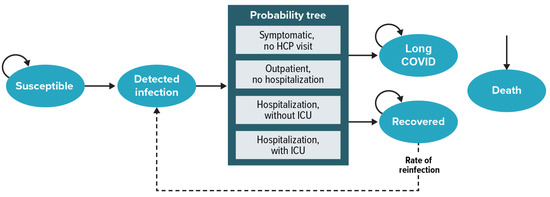
Figure 1
Open AccessArticle
Immunogenicity of Intradermal Versus Intramuscular BNT162b2 COVID-19 Booster Vaccine in Patients with Immune-Mediated Dermatologic Diseases: A Non-Inferiority Randomized Controlled Trial
by
, , , , , , , , , , and
Vaccines 2024, 12(1), 73; https://doi.org/10.3390/vaccines12010073 - 11 Jan 2024
Abstract
The intradermal route has emerged as a dose-sparing alternative during the coronavirus disease 2019 (COVID-19) pandemic. Despite its efficacy in healthy populations, its immunogenicity has not been tested in immune-mediated dermatologic disease (IMDD) patients. This assessor-blinded, randomized-controlled, non-inferiority trial recruited patients with two
[...] Read more.
The intradermal route has emerged as a dose-sparing alternative during the coronavirus disease 2019 (COVID-19) pandemic. Despite its efficacy in healthy populations, its immunogenicity has not been tested in immune-mediated dermatologic disease (IMDD) patients. This assessor-blinded, randomized-controlled, non-inferiority trial recruited patients with two representative IMDDs (i.e., psoriasis and autoimmune bullous diseases) to vaccinate with fractionated-dose intradermal (fID) or standard intramuscular (sIM) BNT162b2 vaccines as a fourth booster dose under block randomization stratified by age, sex, and their skin diseases. Post-vaccination SARS-CoV-2-specific IgG and interferon-γ responses measured 4 and 12 weeks post-intervention were serological surrogates used for demonstrating treatment effects. Mean differences in log-normalized outcome estimates were calculated with multivariable linear regression adjusting for their baseline values, systemic immunosuppressants used, and prior COVID-19 vaccination history. The non-inferiority margin was set for fID to retain >80% immunogenicity of sIM. With 109 participants included, 53 received fID (all entered an intention-to-treat analysis). The fID demonstrated non-inferiority to sIM in humoral (mean outcome estimates of sIM: 3.3, ΔfID-sIM [mean, 95%CI]: −0.1, −0.3 to 0.0) and cellular (mean outcome estimates of sIM: 3.2, ΔfID-sIM [mean, 95%CI]: 0.1, −0.2 to 0.3) immunogenicity outcomes. Two psoriasis patients from the fID arm (3.8%) developed injection-site Koebner’s phenomenon. Fewer fID recipients experienced post-vaccination fever (fID vs. sIM: 1.9% vs. 12.5%, p = 0.027). The overall incidence of disease flare-ups was low without a statistically significant difference between groups. The intradermal BNT162b2 vaccine is a viable booster option for IMDD patients troubled by post-vaccination fever; its role in mitigating the risk of flare-ups remains unclear.
Full article
(This article belongs to the Special Issue Population Immunity and Persistence to SARS-CoV-2 Induced by Infection and Vaccination, Protection against New Infections and Implication for Further Vaccination)
►▼
Show Figures
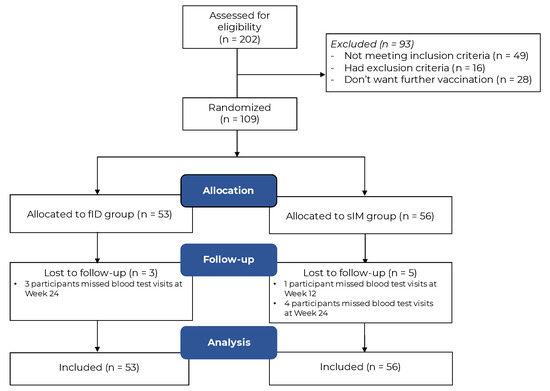
Figure 1
Open AccessArticle
Differences in the Evolution of Clinical, Biochemical, and Hematological Indicators in Hospitalized Patients with COVID-19 According to Their Vaccination Scheme: A Cohort Study in One of the World’s Highest Hospital Mortality Populations
by
, , , , , , , , , , , , , and
Vaccines 2024, 12(1), 72; https://doi.org/10.3390/vaccines12010072 - 11 Jan 2024
Abstract
COVID-19 vaccines primarily prevent severe illnesses or hospitalization, but there is limited data on their impact during hospitalization for seriously ill patients. In a Mexican cohort with high COVID-19 mortality, a study assessed vaccination’s effects. From 2021 to 2022, 462 patients with 4455
[...] Read more.
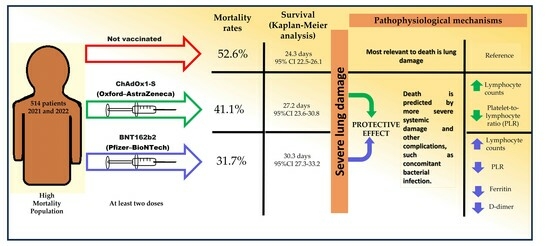
COVID-19 vaccines primarily prevent severe illnesses or hospitalization, but there is limited data on their impact during hospitalization for seriously ill patients. In a Mexican cohort with high COVID-19 mortality, a study assessed vaccination’s effects. From 2021 to 2022, 462 patients with 4455 hospital days were analyzed. The generalized multivariate linear mixed model (GENLINMIXED) with binary logistic regression link, survival analysis and ROC curves were used to identify risk factors for death. The results showed that the vaccinated individuals were almost half as likely to die (adRR = 0.54, 95% CI = 0.30–0.97, p = 0.041). When stratifying by vaccine, the Pfizer group (BNT162b2) had a 2.4-times lower risk of death (adRR = 0.41, 95% CI = 0.2–0.8, p = 0.008), while the AstraZeneca group (ChAdOx1-S) group did not significantly differ from the non-vaccinated (adRR = 1.04, 95% CI = 0.5–2.3, p = 0.915). The Pfizer group exhibited a higher survival, the unvaccinated showed increasing mortality, and the AstraZeneca group remained intermediate (p = 0.003, multigroup log-rank test). Additionally, BNT162b2-vaccinated individuals had lower values for markers, such as ferritin and D-dimer. Biochemical and hematological indicators suggested a protective effect of both types of vaccines, possibly linked to higher lymphocyte counts and lower platelet-to-lymphocyte ratio (PLR). It is imperative to highlight that these results reinforce the efficacy of COVID-19 vaccines. However, further studies are warranted for a comprehensive understanding of these findings.
Full article
(This article belongs to the Special Issue Safety, Efficacy and Optimization of the COVID-19 Vaccines)
►▼
Show Figures
Graphical abstract
Open AccessReview
DNA Vaccines: Their Formulations, Engineering and Delivery
by
and
Vaccines 2024, 12(1), 71; https://doi.org/10.3390/vaccines12010071 - 11 Jan 2024
Abstract
The concept of DNA vaccination was introduced in the early 1990s. Since then, advancements in the augmentation of the immunogenicity of DNA vaccines have brought this technology to the market, especially in veterinary medicine, to prevent many diseases. Along with the successful COVID
[...] Read more.
The concept of DNA vaccination was introduced in the early 1990s. Since then, advancements in the augmentation of the immunogenicity of DNA vaccines have brought this technology to the market, especially in veterinary medicine, to prevent many diseases. Along with the successful COVID mRNA vaccines, the first DNA vaccine for human use, the Indian ZyCovD vaccine against SARS-CoV-2, was approved in 2021. In the current review, we first give an overview of the DNA vaccine focusing on the science, including adjuvants and delivery methods. We then cover some of the emerging science in the field of DNA vaccines, notably efforts to optimize delivery systems, better engineer delivery apparatuses, identify optimal delivery sites, personalize cancer immunotherapy through DNA vaccination, enhance adjuvant science through gene adjuvants, enhance off-target and heritable immunity through epigenetic modification, and predict epitopes with bioinformatic approaches. We also discuss the major limitations of DNA vaccines and we aim to address many theoretical concerns.
Full article
(This article belongs to the Special Issue Feature Papers of DNA and mRNA Vaccines)
►▼
Show Figures
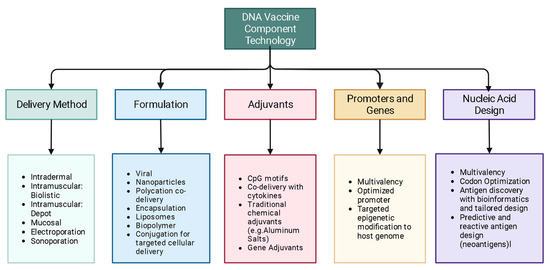
Figure 1
Open AccessArticle
Haemato-Immunological Response of Immunized Atlantic Salmon (Salmo salar) to Moritella viscosa Challenge and Antigens
Vaccines 2024, 12(1), 70; https://doi.org/10.3390/vaccines12010070 - 10 Jan 2024
Abstract
Winter ulcer disease is a health issue in the Atlantic salmonid aquaculture industry, mainly caused by Moritella viscosa. Although vaccination is one of the effective ways to prevent bacterial outbreaks in the salmon farming industry, ulcer disease related to bacterial infections is
[...] Read more.
Winter ulcer disease is a health issue in the Atlantic salmonid aquaculture industry, mainly caused by Moritella viscosa. Although vaccination is one of the effective ways to prevent bacterial outbreaks in the salmon farming industry, ulcer disease related to bacterial infections is being reported on Canada’s Atlantic coast. Here, we studied the immune response of farmed immunized Atlantic salmon to bath and intraperitoneal (ip) M. viscosa challenges and evaluated the immunogenicity of M. viscosa cell components. IgM titers were determined after infection, post boost immunization, and post challenge with M. viscosa. IgM+ (B cell) in the spleen and blood cell populations were also identified and quantified by 3,3 dihexyloxacarbocyanine (DiOC6) and IgM-Texas red using confocal microscopy and flow cytometry. At 14 days post challenge, IgM was detected in the serum and spleen. There was a significant increase in circulating neutrophils 3 days after ip and bath challenges in the M. viscosa outer membrane vesicles (OMVs) boosted group compared to non-boosted. Lymphocytes increased in the blood at 7 and 14 days after the ip and bath challenges, respectively, in OMVs boosted group. Furthermore, a rise in IgM titers was detected in the OMVs boosted group. We determined that a commercial vaccine is effective against M. viscosa strain, and OMVs are the most immunogenic component of M. viscosa cells.
Full article
(This article belongs to the Special Issue Fish Immunology, Vaccines and Novel Treatments)
►▼
Show Figures
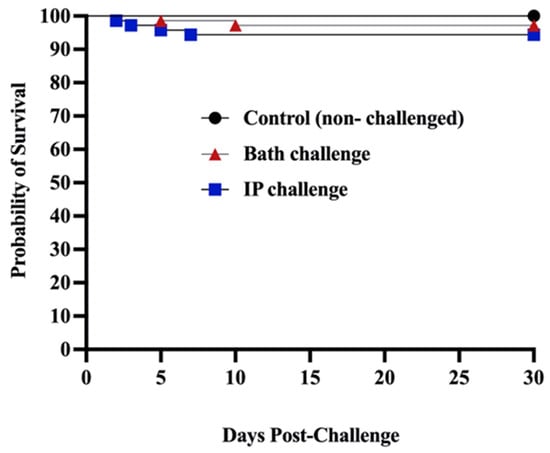
Figure 1
Open AccessArticle
No Waning of Pneumococcal Vaccine Responses over Time in People with Inflammatory Arthritis: Findings from a Single Centre Cohort
by
, , , , , , , , , , , , , , and
Vaccines 2024, 12(1), 69; https://doi.org/10.3390/vaccines12010069 - 10 Jan 2024
Abstract
Background: Vaccination against pneumococcus reduces the risk of infective events, hospitalisation, and death in individual with inflammatory arthritis, particularly in those on immunomodulating therapy who are at risk of worse outcomes from pneumococcal disease. The objective of this study was to investigate the
[...] Read more.
Background: Vaccination against pneumococcus reduces the risk of infective events, hospitalisation, and death in individual with inflammatory arthritis, particularly in those on immunomodulating therapy who are at risk of worse outcomes from pneumococcal disease. The objective of this study was to investigate the serological protection following vaccination against pneumococcal serovars over time. Methods: This was a single centre, retrospective cohort study of individuals with rheumatoid arthritis, psoriatic arthritis, or axial spondylarthritis who had previously received the PPSV23 polysaccharide pneumococcal vaccine (Pneumovax). Data were retrieved between January 2021 to August 2023. Dates of previous pneumococcal vaccination were identified using linked primary care records. Serum serotype levels were collected. The primary outcome was serological response defined as a titre ≥0.35 mcg/mL in at least five from a total of 12 evaluated pneumococcal serovars, examined using a Luminex platform. Multivariate logistic regression models adjusting for age, gender, ethnicity, co-morbidities, and the use of prednisolone, conventional synthetic and biological DMARDs were used to determine the odds of a sustained serological response according to time categorised into ≤5 years, 5–10 years, and ≥10 years since vaccination. Results: Serological response was measured in 296 individuals with inflammatory arthritis, with rheumatoid arthritis the most common diagnosis (74% of patients). The median time between pneumococcal vaccine administration and serological assessment was 6 years (interquartile range 2.4 to 9.9). A positive serological response to at least 5 serovars was present in 195/296 (66%) of patients. Time since vaccination did not significantly associate with serological protection compared with those vaccinated <5 years, the adjusted ORs of vaccine response was 1.15 (95% CI 0.64 to 2.07) in those 5–10 years and 1.26 (95% CI: 0.64 to 2.48) in those vaccinated over 10 years ago. No individual variable from the multivariate model reached statistical significance as an independent predictor of vaccine response, although steroid use at the time of vaccine had a consistent detrimental impact on serological immunity. Conclusions: We demonstrated that antibody titres following vaccination against pneumococcal serovars do not appear to wane over time. It appears more critical to focus on maximising the initial vaccine response, which is known to be diminished in this patient population.
Full article
(This article belongs to the Section Innate and Adaptive Immunity in Vaccination)
►▼
Show Figures
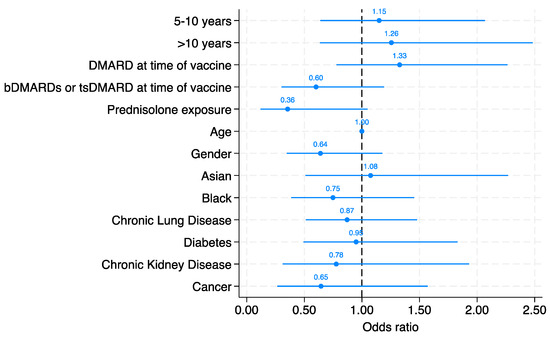
Figure 1

Journal Menu
► ▼ Journal Menu-
- Vaccines Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Diseases, IJMS, Microbiology Research, Pathogens, Vaccines
Advances in Human Pathogen Control—a 21st Century Challenge 2.0
Topic Editors: Jorge H. Leitão, Nitin Amdare, Joana R FelicianoDeadline: 30 June 2024
Topic in
Cells, Diseases, Healthcare, IJMS, Vaccines
Inflammation: The Cause of all Diseases 2.0
Topic Editors: Vasso Apostolopoulos, Jack Feehan, Vivek P. ChavdaDeadline: 31 July 2024
Topic in
Biomedicines, JCM, Pathogens, Vaccines, Viruses
Discovery and Development of Monkeypox Disease Treatments
Topic Editors: Mohd Imran, Ali A. RabaanDeadline: 31 August 2024
Topic in
Brain Sciences, Clinics and Practice, COVID, Life, Vaccines, Viruses
Multifaceted Efforts from Basic Research to Clinical Practice in Controlling COVID-19 Disease
Topic Editors: Yih-Horng Shiao, Rashi OjhaDeadline: 30 September 2024

Conferences
Special Issues
Special Issue in
Vaccines
Bacterial Vaccine: Mucosal Immunity and Implications
Guest Editors: Amit K. Singh, Raj KumarDeadline: 20 January 2024
Special Issue in
Vaccines
Feature Papers of DNA and mRNA Vaccines
Guest Editor: Jorge H. LeitãoDeadline: 31 January 2024
Special Issue in
Vaccines
Advances in COVID-19 Vaccines and Neutralizing Antibody
Guest Editors: Rishi Jaiswal, Srijani Basu, Suman Gupta, Sneh Lata GuptaDeadline: 10 February 2024
Special Issue in
Vaccines
COVID-19 Vaccination, Role of Vaccines and Global Health
Guest Editors: Evridiki Patelarou, Enkeleint A. MechiliDeadline: 29 February 2024
Topical Collections
Topical Collection in
Vaccines
COVID-19 Vaccine Hesitancy: Correlates and Interventions
Collection Editors: Manoj Sharma, Kavita Batra
Topical Collection in
Vaccines
Topic Advisory Panel Members’ Collection Series: Immunization and Vaccines for Infectious Diseases
Collection Editors: Shumaila Hanif, Ravinder Kumar
Topical Collection in
Vaccines
Research on Monoclonal Antibodies and Antibody Engineering
Collection Editor: Tatsuya Yamazaki




_9.50.52.png)